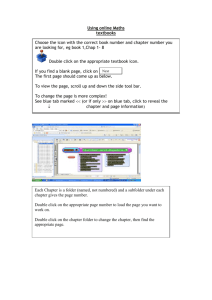
Ch0as - Joseph Smyth
advertisement

<!OPS, Version = 8.0> <!Page Number Stream, Name = Starting Page # = "page", Inherit> <!Document, Header Page = no, Final Output Device = "ps", Default Printer = "nearest-ps", Default Page Stream Name = "page"> <!Font Definitions, F2 = Helvetica 16 Bold, F3 = Times 10, F4 = Helvetica 11, F5 = Helvetica 11 Bold, F6 = Helvetica 12 Bold, F7 = Symbol_A 12, F8 = Helvetica 11 Italic> <!Page, Left Margin = Right Margin = 1 Inches, 1 Inches> <!Autonumber Stream, "list", 1> <!Class, "Authors", Bottom Margin = Alignment = Font = Line Spacing = Left Tab = 0 Inches, Center, F6@Z7@Lam, 1.3104 lines, 0/0.75*13 Inches> Joseph R. Smyth <HR> University of Colorado David L. Bish <HR> Los Alamos National Laboratory <!Class, "Head01", Top Margin = Alignment = Font = Line Spacing = Allow Page Break Allow Page Break Left Tab = 0.14 Inches, Left, F5@Z7@Lam, 1.3114 lines, Within = no, After = no, 1.08 Inches> <!Class, "Head02", Bottom Margin = 0 Inches, Alignment = Left, Font = F3@Z7@Lam, Line Spacing = 1.31 lines, Allow Page Break Within = no, Allow Page Break After = no, Left Tab = <!Class, "Head03", Bottom Margin = Left Margin = First Indent = Alignment = Font = Line Spacing = Allow Page Break Allow Page Break Left Tab = <!Class, "Head04", Left Margin = Alignment = Font = Line Spacing = Allow Page Break Allow Page Break Left Tab = <!Class, "Head05", Bottom Margin = Left Margin = First Indent = Alignment = Font = Line Spacing = Allow Page Break Allow Page Break Left Tab = <!Class, "Head06", Left Margin = Alignment = Font = Line Spacing = Allow Page Break Allow Page Break Left Tab = <!Class, "Head07", Top Margin = Alignment = Font = Line Spacing = Allow Page Break Allow Page Break Left Tab = <!Class, "Para01", Top Margin = Bottom Margin = 0/0.75*13 Inches> 0 Inches, 1 Inches, -1 Inches, Left, F3@Z7@Lam, 1.31 lines, Within = no, After = no, 0/0.75*13 Inches> 0.67 Inches, Left, F3@Z7@Lam, 1.31 lines, Within = no, After = no, 5.33 Inches> 0 Inches, 0.67 Inches, -0.67 Inches, Left, F3@Z7@Lam, 1.31 lines, Within = no, After = no, 0/0.75*13 Inches> 0.67 Inches, Left, F3@Z7@Lam, 1.31 lines, Within = no, After = no, 0/0.75*13 Inches> 0.14 Inches, Left, F3@Z7@Lam, 1.31 lines, Within = no, After = no, 0/0.75*13 Inches> 0.07 Inches, 0.07 Inches, First Indent = Font = Line Spacing = Left Tab = <!Class, "Para02", Left Margin = Alignment = Font = Line Spacing = Left Tab = <!Class, "Para03", Bottom Margin = Left Margin = First Indent = Alignment = Font = Line Spacing = Left Tab = <!Class, "Title", Bottom Margin = Alignment = Font = Line Spacing = Left Tab = 0.24 Inches, F4@Z7@Lam, 1.6001 lines, 0/0.75*13 Inches> 0.67 Inches, Left, F3@Z7@Lam, 1.31 lines, 0/0.75*13 Inches> 0 Inches, 0.67 Inches, -0.67 Inches, Left, F3@Z7@Lam, 1.31 lines, 0/0.75*13 Inches> 0 Inches, Center, F2@Z7@Lam, 1.3109 lines, 0/0.75*13 Inches> Crystal Structures and Cation Sites of the Principal Rock-Forming Minerals <!Master Frame, Name = "floating", Placement = Following Anchor, Horizontal Alignment = Center, Width = 2 Inches, Width = Page Without Margins, Height = 1 Inches, Height = Page Without Margins * 0.33, Diagram = V11, (g9,0,0,)> <Page Header, Frame = V11, (g9,1,0, (t14,1,0,,0,0.4345931,0,7,0,0,,wst:timsps10,) (t14,2,0,,3.25,0.4345931,1,7,0,0,,wst:timsps10,) (t14,3,0,,6.5,0.4345931,2,7,0,0,,wst:timsps10,))> <Page Footer, Frame = V11, (g9,0,0,)> <!End Declarations> <"Title"> <|,"17">Crystal Structures and Cation Sites of the Principal <SR> Rock-Forming Minerals <"Authors"> Joseph R. Smyth <HR> University of Colorado <HR> David L. Bish <HR> Los Alamos National Laboratory <"Para01"> CONTENTS <"Para01"> Acknowledgement iii <HR> Introduction 1 <"Head01"> Unit Cell Tables 2 <HR> Site Data Tables 3 <HR> Systematics of Site Parameter Variation Trace and Minor Element Substitutions 7 5 <HR> <"Head02"> Chapter 1. Single Oxides 10 <"Head01", Bottom Margin = Section 11<HR> Section 14<HR> Section 17<HR> Section Section Section 27<HR> Section Section 34<HR> Section Section Section Section 0 Inches> 1.1<Tab>Cuprite Group 1.2<Tab>Periclase Group 1.3<Tab>Zincite Group 1.4<Tab>Tenorite and Montroydite 1.5<Tab>Corundum Group 1.6<Tab>Bixbyite Group 1.7<Tab>Arsenic and Antimony Sesquioxides 1.8<Tab>Rutile Group 1.9 <Tab> TiO2 Polymorphs 1.10 <Tab>MnO2 Polymorphs 41 <HR> 1.11 <Tab>Uraninite Group 44 <HR> 1.12 <Tab>TeO2 Polymorphs 47 20<HR> 24<HR> 30<HR> 37 <HR> <"Head02"> Chapter 2. Double Oxides 51 <"Head01"> Section 52<HR> Section Section Section Section 2.1<Tab>Ilmenite Group 2.2<Tab>Perovskite 2.3<Tab>Oxide Spinel Group 2.4<Tab>Pseudobrookite Group 2.5<Tab>Tungstate Group 56<HR> 59<HR> 63<HR> 67 <"Head07"> Chapter 3. Hydroxides 72 <"Head03"> Chapter 4.<Tab>Orthosilicates 79 <"Head01"> Section 4.1<Tab>Garnet Group 80<HR> Section 4.2<Tab>Olivine Group 85<HR> <|,"18">Section 4.3<Tab>Silicate Spinel Group 90<HR> Section 4.4<Tab>Silicate Zircon Group 94<HR> Section 4.5<Tab>Willemite Group Section 4.6<Tab>Aluminosilicate Group Section 4.7<Tab>Humite Group 108<HR> Section 4.8<Tab>Titanite Group 117<HR> Section 4.9<Tab>Staurolite 120 98<HR> 102<HR> <"Head02"> Chapter 5. Soro- and Cyclo-silicates 124 <"Head04", Bottom Margin = Section 5.1 Section 5.2 0 Inches> Epidote Group<Tab>125<HR> Melilite Group<Tab>130 <"Head01"> Section 5.3<Tab>Beta Spinel Group 135<HR> Section 5.4<Tab>Lawsonite 139<HR> Section 5.5<Tab>Tourmaline Section 5.6<Tab>Vesuvianite 146 142<HR> <"Head02"> Chapter 6. Chain Silicates 152 <"Head01"> Section 153<HR> Section Section 164<HR> Section Section Section 6.1<Tab>Orthopyroxenes and Primitive Clinopyroxenes 6.2<Tab>C-centered Clinopyroxenes 6.3<Tab>Pyroxenoids 159<HR> 6.4<Tab>Orthoamphiboles 6.5<Tab>Clinoamphiboles 6.6<Tab>Aenigmatite 175<HR> 178<HR> <"Head03"> Chapter 7.<Tab>Layer Silicates 183 <"Head06"> Section 7.1 Talc and Pyrophyllite 184 Section 7.2 Trioctahedral Micas 189 Section 7.3 Dioctahedral <SR> Micas 194 Section 7.4 Clays 199 <"Head03"> Chapter 8.<Tab>Tectosilicates 204 <"Head01"> Section 205<HR> Section Section Section Section 225<HR> Section Section 234 8.1<Tab>Silica Minerals 8.2<Tab>Alkali Feldspar Group 8.3<Tab>Alkaline Earth Feldspars 8.4<Tab>Feldspathoid Group 8.5<Tab>Beryl and Cordierite 212<HR> 216<HR> 220<HR> 8.6<Tab>Scapolite Group 8.7<Tab>Zeolites 230<HR> <"Para03", Bottom Margin = 0.14 Inches> Chapter 9. Carbonates, Nitrates, Sulfates, and Phosphates 262 <HR> Section 9.1 Calcite group 263 <HR> Section 9.2 Dolomite Group 267 <HR> Section Section Section Section Section 9.3 9.4 9.5 9.6 9.7 Aragonite Group 270 <HR> Barite Group 274 <HR> Gypsum and Anhydrite 278 <HR> Apatite 283 <HR> Monazite 287 <"Head05"> <|,"19">Chapter 10. Halides 290 <"Head04"> Section 10.1. Halite Group<Tab>291<HR> Section 10.2. Fluorite Group<Tab>293 <"Para03"> Chapter Section Section Section Section Section Section 11. Cation Sites Listed by Mean Distance. 295 <HR> 11.1 Two- and Three-fold Sites 296 <HR> 11.2 Four-fold Sites 297 <HR> 11.4 Five-fold Sites 303 <HR> 11.5 Six-fold Sites 304 <HR> 11.6 Eight-fold Sites 309 <HR> 11.7 Site of C.N. > 8 310 <"Para03"> References 311 <"Para03"> Mineral Index 319 <"Title", Begin New Page = yes> <|,"20">Crystal Structures and Cation Sites of the Principal <SR> Rock-Forming Minerals <"Authors"> Joseph R. Smyth <HR> University of Colorado <HR> David L. Bish <HR> Los Alamos National Laboratory <HR> <"Head01"> Introduction <"Para01"> Over the past two decades, with the advent of automated x-ray and neutron single-crystal <SR> diffractometers, there has been a major improvement in the precision with which atom positions in <SR> minerals are known. Shannon and Prewitt (1969, 1970), Whittaker and Muntus (1970), and <SR> Shannon (1976) have compiled crystal structure data for synthetic compounds and minerals in <SR> order to estimate effective ionic radii. These compilations and estimates have proven immensely <SR> useful to geochemists, mineralogists, and petrologists in understanding the substitution behavior <SR> and distribution of elements in natural systems, eg. Onuma et al. (1968), Jensen (1973), Philpotts <SR> (1978). Whereas Bragg et al. (1965) and Zoltai and Stout (1984) have compiled descriptions of <SR> mineral structures, and Wyckoff (1963) has compiled atom location data for most inorganic <SR> structures, there has never been a compilation of data on the nature of cation sites in minerals. <"Para01"> Robie et al. (1978) compiled thermodynamic data for many of the rockforming minerals and <SR> oxides. For some of these compounds there have been more recent and accurate cell <SR> determinations, so that improved data on molar volume and density are available. Further, <SR> thermodynamic data compilations do not include information on atomic environments in these <SR> compounds. .PP We have undertaken a compilation of recent data on crystal structures for a large <SR> group of the common minerals. From atom positional and cell data, we have calculated <SR> nearest-neighbor distances, coordination numbers, volumes of coordination polyhedra, distortion <SR> indices, and electrostatic energies in a consistent fashion. The objective in this work is to make the <SR> recent improvements in crystal structure data available to a larger group of petrologists and <SR> geochemists seeking to understand the chemical behavior of these minerals in natural systems. <"Para01"> In order to reduce this to a manageable task we have had to make some rather arbitrary <SR> decisions in selecting and grouping the data. First, we have limited the the group to the oxygen and <SR> halide minerals with the understanding that ionic radii have at least some relevance to these <SR> structures. This has led to the exclusion of the sulfides from the current compilation. In selecting <SR> structures, we have endeavored to choose ordered end-members whenever possible so that <SR> cation site data will be more easily interpretable. In order to document atomic environments in <SR> standard thermodynamic states we have included a large number of simple oxide minerals. This <SR> <|,"21">has led to the inclusion of some less-than-common minerals in this group, but otherwise we have <SR> included only the more common minerals of igneous, metamorphic, and sedimentary rocks. Finally, <SR> in order to facilitate comparisons, we have grouped together data from isomorphous structures, and <SR> in a few cases, polymorphous structures. This has led to a few instances of duplication which we feel <SR> are justified in order to allow comparisons. <"Head01"> Unit Cell Tables <"Para01"> We have organized the structure data into those pertaining to unit cells and those pertaining to <SR> specific sites. In addition, we have summarized the site data, grouped them according to <SR> coordination number, and listed them by mean distance in Chapter 11. Within the mineral groups, <SR> unit cell tables consist of formula, formula weight, calculated density, molar volume, Z, crystal <SR> system, class, and space group, cell parameters, and reference. In general, the formula is that given <SR> in the reference, except that we have omitted elements constituting less than 1.0 weight percent of <SR> the mineral. In a few instances we have recalculated formulas to the same number of oxygens for <SR> comparison across an isomorphous series. The formula weight, density, and molar volume are our <SR> calculation from the stated formula and cell. Z is the number of formula units per cell. The reference <SR> is not repeated in the site tables, but site data are presented sequentially in the same order <SR> permitting unambiguous citation. <"Head01"> Site Data Tables <"Para01"> Similar sites in isomorphous series are grouped together to facilitate comparisons and show <SR> variability of analogous features across the series. The tabulated data consist of a site name, <SR> coordination number (C.N.), occupants, point symmetry, Wyckoff notation, fractional coordinates, <SR> nearest neighbor distances, mean and standard deviation of distances, polyhedral volume, <SR> quadratic elongation, variance of central angle, electrostatic site energy, and a model charge. The <SR> coordination number is the number of nearest anion neighbors. The occupant is that inferred from <SR> the formula or stated in the reference. In a few instances, for partially occupied sites, a total site <SR> occupancy is given. Tetrahedral Al-Si occupancies for some of the zeolites were calculated from <SR> the mean T-O distance when site occupancies were not reported. The point symmetry and Wyckoff <SR> notation are those for the site (Hahn, 1983). Fractional coordinates for the site are included to avoid <SR> any ambiguities in site nomenclature that may arise and to show variability across the series. <SR> Individual nearest neighbor distances are given throughout with a major exception being those for <SR> the framework silicate structures (Chapter 8). With the low symmetries of many of these structures, <SR> it was found very difficult to present these in a way that would be both concise and meaningful. Also, <SR> we have omitted the cavity geometries for the zeolites as these are documented elsewhere (Mortier, <SR> 1982). <"Para01"> <|,"22">The mean distance is our calculated average of the given distances. The<F7@Z7@Lam> s<F0> is the standard <SR> deviation of the distances. It is thus an estimate of the distortion of the site, not an estimate of the <SR> error in the determination. Errors are regrettably not given because this would have more than <SR> doubled the size of the data base, greatly complicating the handling of the data. The polyhedral <SR> volume (Poly. Vol.), quadratic elongation (Q.E.), and angle variance (Ang.Var.) were calculated with <SR> a slightly modified version of the program VOLCAL (L.W. Finger, personal communication). The <SR> units of polyhedral volume are cubic Angstroms. Quadratic elongation as defined by Robinson et al. <SR> (1971) is unitless, and the units of the variance of the central polyhedral angle are degrees squared. <SR> These two quantities are defined only for octahedra and tetrahedra. The electrostatic site energy <SR> was calculated with the program ELEN (Y. Ohashi, personal communication). Calculations were <SR> performed on a VAX 11/750 computer using double-precision arithmetic. High symmetry <SR> structures were reduced to triclinic symmetry. The units are kcal/mole and are the amount of <SR> electrostatic energy derived by placing 1 mole of cations of the stated charge into the site, assuming <SR> a purely point charge model. This energy is recalculated as electron volts (eV) in Chapter 11. The <SR> model charge is that used for the electrostatic calculation, however this may be omitted in instances <SR> where it is an integer unambiguously inferred from site occupants. These energies are included for <SR> qualitative comparisons among sites and should not be used for quantitative calculations because <SR> they exclude repulsive forces entirely. In addition, partial charges do not accurately model the <SR> effects of disorder. <"Head01"> Systematic Variation of Site Parameters <"Para01"> With a data base of this size, it is relatively straightforward to examine correlations between <SR> various site parameters. Such correlations help explain the nature of variations seen from structure <SR> to structure. Two particularly useful correlations are between angle variance and quadratic <SR> elongation and between electrostatic potential and mean cation-anion distance for individual sites. <"Para01"> <F8@Z7@Lam>Distortions.<F0>There are several parameters that can be used as indicators of distortions or <SR> regularity of coordination polyhedra. For regular tetrahedra and octahedra, the angular distortions <SR> are conveniently indicated by the variance of the central angle (Ang.Var.). In addition to angular <SR> distortion is distance distortion, a convenient measure of which is the standard deviation of the <SR> distances ($sigma$) which can be used to indicate distortion of cations of any coordination number. <SR> A factor called quadratic elongation (Q.E.) (Robinson et al., 1971) is also calculated for each <SR> octahedron and tetrahedron and is a convenient measure of both angular and distance distortions. <SR> Figure 0.1 is a plot of angle variance versus quadratic elongation for a large number of tetrahedra <SR> and shows that the two parameters are, of course, strongly correlated. There are a few instances in <SR> which the angle variance plots well below the trend, but no instances in which it plots above. This is <SR> <|,"23">likely due to the fact that in a few instances, such as for a tetrahedron on three-fold axis, the angles <SR> may be more strongly constrained by symmetry than the distances. <"Para01"> <F8@Z7@Lam>Electrostatic Energies.<F0> It is also of interest to examine the variation of electrostatic energy with <SR> distance. The electrostatic energy reported in the site tables is in kcal/mole of sites. In Chapter 11, <SR> these energies are converted to electron-volts and divided by the charge to give a potential in volts. <SR> The total electrostatic energy of the crystal would then be half the sum for all sites in the formula unit. <SR> The total electrostatic energies calculated for each of these mineral structures is adequate to allow <SR> full ionization of all species to their normal valences, if reasonable allowances for repulsion energies <SR> are included in Born-Haber calculations. <"Para01"> The electrostatic energy is by no means the total energy of the crystal. It specifically excludes <SR> nearest-neighbor repulsion energies which may be ten percent or more of total energy. In addition, <SR> it excludes any estimate of distortion energies of electron distributions (e.g. crystal field stabilization <SR> energies) and energies of thermal vibrations. The energies cannot be used to compute heats of <SR> formation or predict relative thermodynamic stability of various polymorphs because the energy <SR> differences between polymorphs is typically much smaller than the excluded terms. The energies <SR> are, however, useful and instructive for qualitative comparisons between sites. Further, much <SR> progress has been made in recent years on prediction of mineral structures based on electrostatic <SR> energies combined with simple to complex expressions for nearest-neighbor repulsion energies <SR> (e.g., Catlow et al., 1982, Price and Parker, 1984). <"Para01"> We have plotted electrostatic potential (eV/chg) versus mean cation-anion distance for some <SR> 700 sites (Figure 0.2) and observe a very strong correlation. This figure shows clearly that there is a <SR> systematic electrostatic contribution to the energy of the crystals. Further, we have preliminary <SR> indication that deviations from the observed trend are significant and potentially useful indicators of <SR> minor element substitutions. <"Head01"> Trace and Minor Element Substitutions <"Para01"> One of the major reasons for undertaking this compilation was to provide geochemists with a <SR> convenient and comprehensive source of information on mineral sites. We hope that it will be of use <SR> in understanding trace element and minor element distributions in geochemical systems. We have <SR> noted a few interesting correlations and general observations that are worth mentioning here. We <SR> hope that users of this volume will find many more. <"Para01"> The electrostatic energy and its variation with mean cation-anion distances may be a potentially <SR> useful indicator of minor element substitution sites. For example, of the two large cation sites in the <SR> epidote group (5.1), the A2 site has the much deeper electrostatic potential well despite its larger <SR> <|,"24">volume. It has, in fact the largest electrostatic energy per charge (potential) of any site surveyed with <SR> coordination number greater than 8 (Table 11.7). This accounts for the preference of trivalent rare <SR> earth elements for the A2 site over the A1 site in allanite and may explain why allanite has been <SR> observed to have distribution coefficients for rare earths relative to whole rocks of 1000 or more. It is <SR> relatively straightforward, then to identify potential sites for lanthanides and actinides from Tables <SR> 11.6 and 11.7. <"Para01"> We have also noted that sites tend to favor minor element substitutions that minimize distortions <SR> of the site or of the mineral as a whole, that is, of the other sites in the mineral as well. Recent <SR> geochemical studies have noted that the rhombohedral carbonates siderite and calcite both show <SR> large distribution coefficients for Mn relative to aqueous fluids (Ishikuni, 1984). In plotting distortion <SR> coefficients of the octahedral site versus mean distance in pure carbonates, we see a minimum in <SR> the distortion, particularly in angle variance, near Mn. This is in marked contrast to most silicate <SR> octahedral sites which show a preference for smaller cations and may reflect a smaller effective <SR> radius for oxygen in the silicate octahedra relative to the carbonate octahedra. This may also be true <SR> for a broad range of octahedral sites in silicates and may account for the observation of Goldschmidt <SR> (1958) that in general mineral sites prefer smaller, rather than larger, cations. We note that for many <SR> silicates (e.g. olivine and orthopyroxene) the distortions of the octahedral sites decrease with radius <SR> at least down to the radius of Ni and that these structures have a strong preference for smaller <SR> cations in these sites. <"Para01"> The preference for smaller cations by silicate sites is certainly not applicable to sites other than <SR> regular octahedra. The X site in garnet is a fine example. It may contain Ca, Mn, Fe, or Mg and is <SR> highly symmetric with point symmetry 222. Its only measure of distortion, <F7@Z7@Lam>s<F0>, does not vary strongly <SR> with cation radius, but the distortion of the Si site decreases strongly with increasing X-site radius. <SR> We would predict from these considerations then that if garnet crystallized as a liquidus phase, as it <SR> apparently does in the eclogite system, it would preferentially accept Ca, then Fe, then Mg, and thus <SR> possibly enrich residual liquids in Mg. <"Para01"> We have included these discussions on major and trace element distributions to encourage <SR> users of these tables to look for correlations between crystal structure parameters and element <SR> distributions in natural systems. It is our sincere hope in compiling these data that they will lead to <SR> greater understanding of geochemistry as well as mineralogy. <"Title", Begin New Page = <|,"25">ACKNOWLEDGEMENT <"Para01"> yes> This work was supported in part by the U.S. Department of Energy, Office of Basic Energy <SR> Sciences, through several grants to Los Alamos National Laboratory which is operated by The <SR> University of California under contract number W-7405-ENG-6 The authors particularly thank Dr. <SR> George Kolstadt (OBES - Chemistry, Earth, and Life Sciences) and Dr. Ryszard Gajewski (OBES - <SR> Advanced Energy Projects) for generous support of the project. The authors thank Drs. Y. Ohashi <SR> (ARCO, Plano, TX), R. X. Fischer (Johannes Gutenberg Universitaet, Mainz), and L. W. Finger <SR> (Carnegie Institution, Washington, DC) for providing computer codes and discussions and Drs. <SR> George Zweig, Klaus Lackner, and Wes Myers (Los Alamos National Laboratory) for discussions, <SR> support, and encouragement throughout the project. Theoretical Division Office of Los Alamos <SR> National Laboratory is also thanked for its support, and Tamsin C. McCormick is gratefully <SR> acknowledged for tireless proofreading, technical assistance and moral support.