CP Worksheet - Charges and Coulomb's Law
advertisement

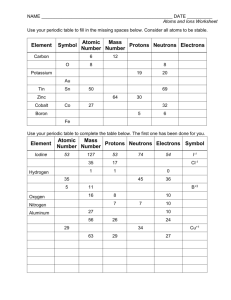
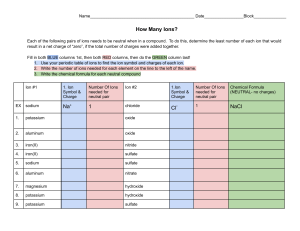
CP Worksheet 136 - Charges and Coulomb’s Law Review CP030412A 1. An ion has a charge of + 3.2 x 1014 C. Another charge is brought near it and experiences a repulsive electrostatic force. This other charge must be a) positive b) negative c) neutral d) either positive or negative 2. An electron has a charge which is a) positive b) negative c) neutral d) either positive or negative 3. A proton has a charge which is a) positive b) negative c) neutral d) either positive or negative 4. A neutron has a charge which is a) positive b) negative c) neutral d) either positive or negative 5. An ion consist of 12 electrons. What is the charge on the ion? 6. An ion has 18 protons and 13 electrons. What is the charge on the ion? 7. An ion has a charge of -9.6 x 10-9 C. How many excess electrons does the ion have? 8. An ion has a charge of 6.4 x 10-17 C. 8.2 x 10-4 meters away is a second charge with a charge of 9.6 x 10-16 C. a. What is the strength of the electrostatic force between the ions? b. Is the force attractive or repulsive? 9. According to the Coulomb’s Law formula, if you double the charge on one of the two ions, how will this affect the electrostatic force between the two ions? a) b) c) d) The FE will be four times large The FE will be one forth as large The FE will be twice as large The FE will be one half as large 10. According to the Coulomb’s Law formula, if you double the distance between two ions, how will this affect the electrostatic force between the ions? a) b) c) d) The FE will be four times large The FE will be one forth as large The FE will be twice as large The FE will be one half as large 11. (Tough one) The force between two protons is 5.5 x 10-15 n. How far apart are the protons?