NO2 – N2O4 Equilibrium: Temperature and LeChatelier's Principle
advertisement

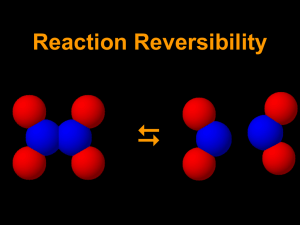
14-1 NO2 – N2O4 Equilibrium: Temperature and LeChatelier’s Principle Sources: Chemical Demonstrations Volume 2 by Bassam Shakhashiri, pp180-83 and Merck Index 11th edition, p 1045. Description: Two ~ 1 ft long glass tubes containing a reddish colored gas and put in a dry-ice-isopropy alcohol bath. The tubes loose their gas and a green liquid condenses on the bottom. When one tube is returned to room temperature, its reddish color is restored. Concept: NO2 gas exists as a mixture of NO2 and N2O4 at equilibrium. It is a reddish color. When the temperature is changed the equilibrium shifts. Cooling the gas, causes a shift towards the dimer, N2O4, which should be colorless when pure but can be blue-green due to impurities such as N2O3. The dimerization is NO2 is exothermic. 2 NO2 N2O4 + heat Materials: Two NO2-N2O4 glass tubes from Sargent-Welch (stored in cabinet in service hallway) One or two dewars Dry-ice Isopropyl alcohol Safety: NO2 gas is extremely toxic. According to Merck Index: “ Inflammation of lungs may cause only slight pain or pass unnoticed, but the resulting edema several days later may cause death”. 200 ppm may be fatal. Please handle the tubes with extreme care. Do not use them to stir the ice bath! Procedure: Before class, prepare an dry-ice-isopropyl alcohol bath or two in the dewars. During class put one or both of the NO2-N2O4 tubes in the bath(s). Observe the color change. Remove one tube from the bath and observe color change. Compare the warmer tube to the colder tube. Clean-up: Remove the tubes from the dry-ice bath, allow to return to room temperature on there own, and then carefully wrap them in bubble wrap, put them to their storage box, and return them to their cabinet in the service hallway. The isopropyl alcohol can be drained from the bath and reused for the next time. 14-1