File

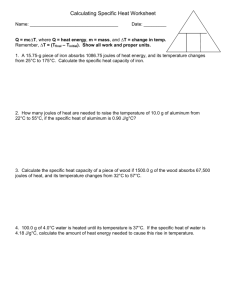
Specific Heat Capacity
Worksheet
Name_______________
Date___________B____
Solve each problem below using these steps :
List data
Re-arrange Equation
Solve
Q
=
m C p
Δ
T
C p
H
2
O = 4.184 J g
C
1. How much thermal energy must be absorbed by 20.0 g of H
2
O to increase its temperature from
283.0 °C to 303.0 °C? [Answer = 1674 J]
List data Re-arrange Equation Solve (estimate & check units)
2.
If it takes 41.72 joules to heat a piece of gold weighing 18.69 g from 10.0 °C to 27.0 °C, what is the specific heat of th e gold? [Answer = 0.13 J/g °C]
List data
Re-arrange Equation
Solve (estimate & check units)
3. A certain mass of water was heated with 40.8 Joules, raising its temperature from 22.0 °C to 28.5
°C. Find the mass of the water in grams. [Answer = 1.50 g]
List data Re-arrange Equation Solve (estimate & check units)
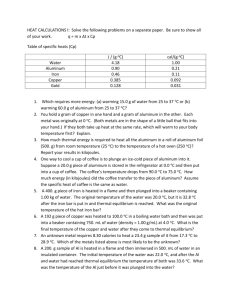
Specific Heat Capacity
Name_______________
Date___________B____
4. The specific heat of ethanol is 2.46 J/g °C. Find the amount of thermal energy required to raise the temperature of 193 g of ethanol from 19°C to 35°C. [Answer = 7600 J]
List data Re-arrange Equation Solve (estimate & check units)
5. When a 1.2 g sample of aluminum (Al) absorbs 9.61 J of energy, its temperature increases from
25°C to 34°C. Find the specific heat of aluminum. [Answer = 0.89 J / g °C]
List data
Re-arrange Equation
Solve (estimate & check units)
6. The specific heat of Iron is 0.46 J/g °C. A 10 g sample of Iron is heated to a final temp. of 96 °C by absorbing 350 J of energy. What was initial temperature of the Fe sample? [Answer = 20 °C]
List data Re-arrange Equation Solve (estimate & check units)