Isotopes Lab
advertisement

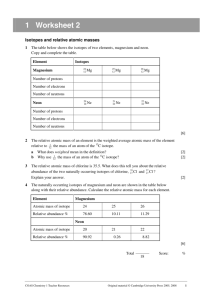
Chapter 4 Chemistry Lab: Isotopes Name: In this lab, you will investigate isotopes. As you recall, isotopes of an element are atoms that have the same number of protons, but have different numbers of neutrons. You will find the number of isotopes of several elements: pennium, brownM&Mium and redM&Mium. You will also find the percent abundance of each of the isotopes. Finally, you will calculate the average atomic mass of the atoms of each type of element. Pennium Find one of the containers containing atoms of pennium. Container letter: _________ Using a balance, find the mass of each of the pennies in the container. Record the year and the mass of each penny in the table below. You might not use all of the spaces. Year Mass (g) Year Mass (g) Year Mass (g) Summary of Data Isotope Description 1 Approx. Mass = 2 Approx. Mass = Number of Atoms Percent Abundance (show work) Calculate the Average Atomic Mass for Each Isotope of Pennium by adding up all of the masses and dividing by the number of atoms of that isotope (show some work or give a brief explanation) ISOTOPE 1 ISOTOPE 2 Calculate the Overall Average Atomic Mass of Pennium by using the Average Atomic Mass of each isotope (calculated in the previous step, above) and the % abundance of each isotope (show work). BrownM&Mium Find one of the containers filled with atoms of brownM&Mium. Container letter: __________ Fill in the table below. Summary of Data Isotope Description 1 Mass = 2 2 Mass = 1 Number of Atoms Percent Abundance (show work) Calculation of Average Atomic Mass for BrownM&Mium (show work) RedM&Mium Find one of the containers filled with atoms of redM&Mium. Container letter: __________ Fill in the table below. Summary of Data Isotope Description 1 Mass = 3 2 Mass = 2 Number of Atoms Percent Abundance (show work) Calculation of Average Atomic Mass for RedM&Mium (show work) ANALYSIS QUESTIONS Refer back to your pennium data and answer the following questions. 1. What do the pennies represent in this investigation? 2. What do the different masses of the pennies represent? 3. What information do you need to calculate the average atomic mass of an element? 4. Was the mass of 20 pennies equal to 20 times the mass of one penny? Explain. 5. In what year did the mass of pennium change? 6. How could you tell? How can you explain that there are different “isotopes” of pennium? Refer back to your M&Mium data and answer the following question. 7. Why was the average atomic mass of red different from the average atomic mass of brown? Applications: Answer the following questions. 8. Why are the atomic masses for most elements NOT whole numbers? 9. How are the 3 isotopes of hydrogen (H-1, H-2, and H-3)… ALIKE DIFFERENT 10. Copper has 2 isotopes, copper-63 and copper-65. The percent abundance of copper-63 is 69.1% and that of copper-65 is 30.9%. Find the average atomic mass of copper. 11. Lithium has 2 isotopes, Li-6 and Li-7. The percent abundance of Li-6 is 7.42% and that of Li-7 is 92.58%. Find the average atomic mass of Li. 12. Silver has 2 isotopes, Ag-107 and Ag-109. The percent abundance of Ag-107 is 51.35% and that of Ag-109 is 48.65%. Find the average atomic mass of silver.