PreLab
advertisement

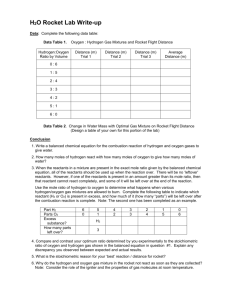
Name Date Period MicroMole Rockets Pre-Lab In this lab, you will use film canisters and pipettes to construct hydrogen and oxygen generators. Read steps 3 and 4 of the procedure. In the space below make a labeled diagram of the oxygen and the hydrogen gas generators. Generating hydrogen: In order to generate hydrogen, we will use a single displacement reaction in which solid zinc reacts with aqueous hydrochloric acid (HCl) to form hydrogen gas and one other product. 1. Write a balanced chemical equation (including states of matter) for the reaction we will be using to generate hydrogen: 2. Ms. Lyall determined that 0.654 g of Zn reacted in her hydrogen generator. How many moles of Zn were consumed in the reaction? Show your work. Use conversion factors and dimensional analysis. 3. Ms. Lyall filled her rocket with 2.5mL of hydrogen gas. How many moles of hydrogen gas did she use? Show your work. Use conversion factors and dimensional analysis. Generating oxygen: In order to generate oxygen, we will catalyze the decomposition of hydrogen peroxide (H2O2) by adding catalase (from yeast), producing liquid water and oxygen gas. 1. Write a balanced chemical equation (including states of matter) for the reaction we will be using to generate oxygen: 2. Mr. Schafer filled his rocket with 1.2mL of oxygen, How many moles of oxygen gas did he use? Show your work. Use conversion factors and dimensional analysis. 3. In a separate trial, Mr. Schafer added 1.5 mL of oxygen to the rocket. What is the mass of the oxygen he added to the rocket? Show your work, conversion factors, and dimensional analysis. Collecting gas and launching your rocket: Make a diagram to show how you will be collecting the gasses in steps 5-8 of the procedure: You will provide a spark to ignite the oxygen – hydrogen mixture. In the reaction, oxygen and hydrogen gases will combine to form water vapor. 1. Write a balanced equation for the reaction 2. Considering your answer to question 1, how many moles of oxygen gas are required in order to consume one mole of hydrogen gas?