Molar Mass Chp
advertisement
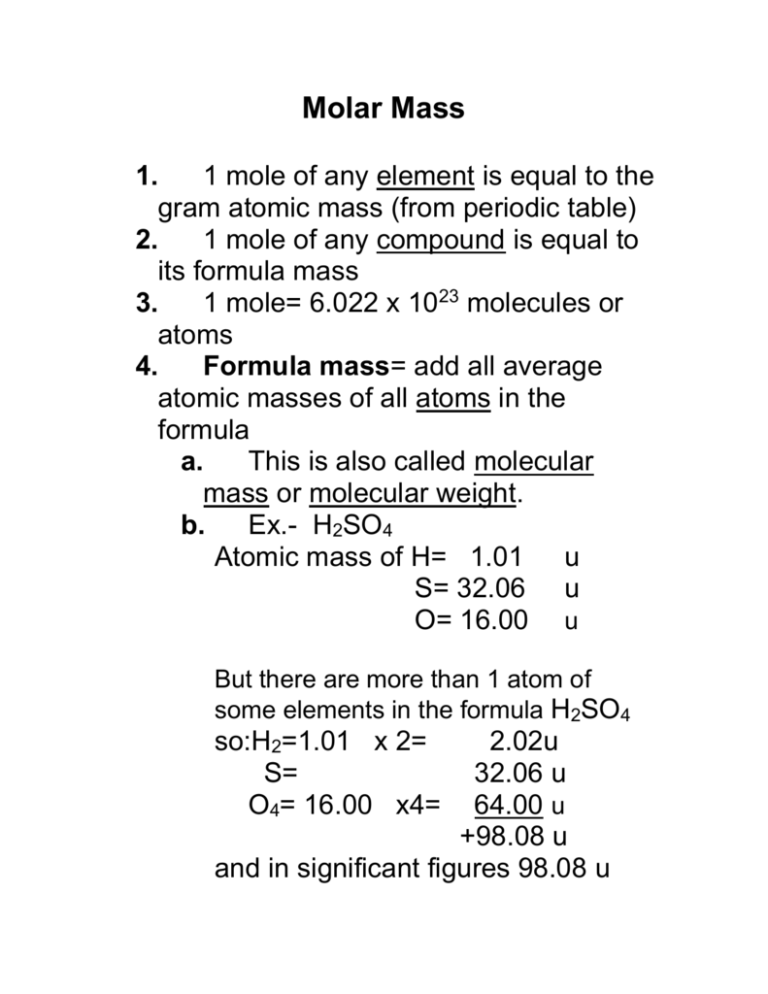
Molar Mass 1. 1 mole of any element is equal to the gram atomic mass (from periodic table) 2. 1 mole of any compound is equal to its formula mass 3. 1 mole= 6.022 x 1023 molecules or atoms 4. Formula mass= add all average atomic masses of all atoms in the formula a. This is also called molecular mass or molecular weight. b. Ex.- H2SO4 Atomic mass of H= 1.01 u S= 32.06 u O= 16.00 u But there are more than 1 atom of some elements in the formula H2SO4 so:H2=1.01 x 2= S= O4= 16.00 x4= 2.02u 32.06 u 64.00 u +98.08 u and in significant figures 98.08 u 5. molar mass= this is the mass of one mole of a compound a. it is calculated the same as formula mass but instead of u (units) use g (units). b. The molar mass of H2SO4 = 98.08g c. One mole of H2SO4 contains 6.022 x 1023 H2SO4 molecules. d.Yet if we look at ions there are 2 moles of H+ ions and 1 mole of SO4-2 ions 3 moles of ions total e. Yet if we look at atoms there are 2 moles of hydrogen 1 mole of sulfur 4 moles of oxygen 7 moles of atoms total 6. Conversions Factors a. 6.022 x 1023 molecules or 1 mol 1 mol 6.022 x 1023 molecules b. moles x grams 1 mole = mass in grams c. mass in grams x 1 mole = moles grams d. ex. What is the mass in grams of 2.5 moles of oxygen gas? (O2) 2.5 mol O2 x 32.0 g O2 1 mole O2 = 80 g O2 mass O from periodic table = 16.00 g O2 will be double = 32.0 g 7. Percent Composition= the percent by mass of each element in the compound. All parts have to total 100%. Mass of element Molar mass of compound Ex. PbCl2 = % of element molar mass Pb = 207.2g Molar mass Cl = 35.453g 2 x 35.453g =70.906g Cl2 Molar mass of PbCl2 then equals 278.106g % Pb = 207.2 g = 0.7451 or 74.51% 278.106g % Cl2 = 70.906g = 0.2550 or 25.50% 278.106g In other words, the % composition = part Total If the percent composition for 2 mystery compounds is the same then the compounds are probably the same. Percent composition does not change whether you have a small or large sample.







