Electron Configurations
advertisement

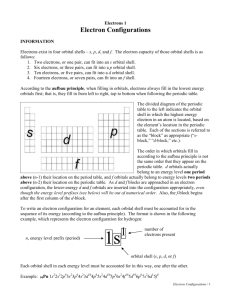
Electrons 1 Electron Configurations INFORMATION Electrons exist in four orbital shells – s, p, d, and f. The electron capacity of those orbital shells is as follows: 1. Two electrons, or one pair, can fit into an s orbital shell. 2. Six electrons, or three pairs, can fit into a p orbital shell. 3. Ten electrons, or five pairs, can fit into a d orbital shell. 4. Fourteen electrons, or seven pairs, can fit into an f shell. According to the aufbau principle, when filling in orbitals, electrons always fill in the lowest energy orbitals first. The divided diagram of the periodic table to the left indicates the orbital shell in which the highest energy electron in an atom is located, based on the element’s location in the periodic table. Each of the sections is referred to as the “block” as appropriate (“s-block,” “d-block,” etc.). The order in which orbitals fill in according to the aufbau principle is not the same order that they appear on the periodic table. d and f orbitals actually belong to energy levels two periods lower than their own energy levels indicate from the periodic table. As d and f blocks are approached in an electron configuration, the lower-energy d and f orbitals are inserted into the configuration appropriately, even though the energy level prefixes (see below) will be out of numerical order. To write an electron configuration for an element, each orbital shell must be accounted for in the sequence of its energy (according to the aufbau principle). The format is shown in the following example, which represents the electron configuration for hydrogen: energy level prefix (period) 1s 1 number of electrons present orbital shell (s, p, d, or f) Each orbital shell in each energy level must be accounted for in this way, one after the other. Electron Configurations / 1 Noble-Gas Abbreviations Electron configurations for high-atomic number elements can be abbreviated using noble gas notation. For example, sodium’s abbreviated electron configuration is: [Ne]3s 1 Elements in period 2 of the periodic table are not abbreviated in this way. Key Questions 1. Write complete electron configurations for: a. Helium e. Silicon i. Bismuth b. Lithium f. Arsenic j. Fermium c. Magnesium g. Iridium k. Roentgenium d. Oxygen h. Uranium 2. Write noble-gas abbreviated electron configurations for the same elements: a. Helium e. Silicon i. Bismuth b. Lithium f. Arsenic j. Fermium c. Magnesium g. Iridium k. Roentgenium d. Oxygen h. Uranium Electron Configurations / 2