Pressure
advertisement

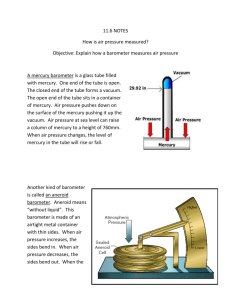
CHAPTER 5:: PRESSURE Introduction kg m F which would have the units of 2 2 . A s m A standard measure of pressure is a Pascal Pa 1 N m2 where 100Pa = 1mb. We consider the follow Pressure is defined as p conversions: 1 atm 1013 .25 mb 760 mm Hg 29 .92 in Hg Although pressure is an absolute quantity, everyday pressure measurements, such as for tire pressure, are usually made relative to ambient air pressure. In other cases measurements are made relative to a vacuum or to some other ad hoc reference. Pressure measurements can be divided into three different categories: absolute pressure, gage pressure, and differential pressure. Absolute pressure refers to the absolute value of the force per-unit-area exerted on a surface by a fluid. Therefore the absolute pressure is the difference between the pressure at a given point in a fluid and the absolute zero of pressure or a perfect vacuum. Gage pressure is the measurement of the difference between the absolute pressure and the local atmospheric pressure. Local atmospheric pressure can vary depending on ambient temperature, altitude and local weather conditions. The U.S. standard atmospheric pressure at sea level and 59°F (20°C) is 14.696 pounds per square inch absolute (psia) or 101.325 kPa absolute (abs). When referring to pressure measurement, it is critical to specify what reference the pressure is related to. A perfect vacuum is zero absolute pressure. Differential pressure is simply the measurement of one unknown pressure with reference to another unknown pressure. The pressure measured is the difference between the two unknown pressures. In addition to the three types of pressure measurements, there are different types of fluid systems and fluid pressures. There are two types of fluid systems; static systems and dynamic systems. As the names imply, a static system is one in which the fluid is at rest and a dynamic system is one in which the fluid is moving. STATIC PRESSURE SYSTEMS The pressure measured in a static system is static pressure. For a uniform static fluid, the pressure is the same at all points along the same horizontal plane in the fluid and is independent of the shape of the container. The pressure increases with depth in the fluid and acts equally in all directions. The increase in pressure at a deeper depth is essentially the effect of the weight of the fluid above that depth. For example, two different containers with the same fluid exposed to the same external pressure. At any equal depth within either tank the pressure will be the same. The pressure is dependent only on depth and has nothing to do with the shape of the container. In a static pressure system, the change of pressure with height can be described and computed by the hydrostatic balance. DYNAMIC PRESSURE SYSTEMS Dynamic pressure systems are more complex than static systems and can be more difficult to measure. In a dynamic system, pressure typically is defined using three different terms. The first pressure we can measure is static pressure. This pressure is the same as the static pressure that is measured in a static system. Static pressure is independent of the fluid movement or flow. As with a static system the static pressure acts equally in all directions. The second type of pressure is what is referred to as the dynamic pressure. This pressure term is associated with the velocity or the flow of the fluid. The third pressure is total pressure and is simply the static pressure plus the dynamic pressure. STEADY-STATE DYNAMIC SYSTEMS Care must be taken when measuring dynamic system pressures. For a dynamic system, under steady-state conditions, accurate static pressures may be measured by tapping into the fluid stream perpendicular to the fluid flow. For a dynamic system, steady-state conditions are defined as no change in the system flow conditions: pressure, flow rate, etc. Although the static pressure is independent of direction, the dynamic pressure is a vector quantity which depends on both magnitude and direction. TRANSIENT SYSTEMS 1 Transient systems are systems with changing conditions such as pressures, flow rates, etc. Measurements in transient systems are the most difficult to accurately obtain. If the measurement system being used to measure the pressure has a faster response time than the rate of change in the system, then the system can be treated as quasi-steady-state. That is, the measurements will be about as accurate as those taken in the steady-state system. Atmospheric pressure measurement The atmospheric pressure must be measured as absolute pressure. Atmospheric pressure is typically about 1000 hPa at sea level, but is variable with altitude and weather. If the absolute pressure of a fluid stays constant, the gauge pressure of the same fluid will vary as atmospheric pressure changes. For example, when a car drives up a mountain, the tire pressure goes up. Some standard values of atmospheric pressure such as 101.325 kPa or 100 kPa have been defined, and some instruments use one of these standard values as a constant zero reference instead of the actual variable ambient air pressure. This impairs the accuracy of these instruments, especially when used at high altitudes. Barometers The mercury barometer was developed in 1643 by Torricolli, and it was the first barometer. He attempted to create a vacuum by inverting a closed tube of mercury into a bowl of mercury. He figured that the weight of the mercury can create a vacuum in the top of the tube (not a complete vacuum though). Mercury in the tube adjusts until the weight of the mercury column balances the atmospheric force exerted on the reservoir. High atmospheric pressure places more force on the reservoir, forcing mercury higher in the column. Low pressure allows the mercury to drop to a lower level in the column by lowering the force placed on the reservoir. Since higher temperature at the instrument will reduce the density of the mercury, the scale for reading the height of the mercury is adjusted to compensate for this effect. Torricelli noticed that the height of the mercury in a barometer changed slightly each day and concluded that this was due to the changing pressure in the atmosphere. He wrote: "We live submerged at the bottom of an ocean of elementary air, which is known by incontestable experiments to have weight". Pascal heard of this experiment, and his brother-in-law Fortin Prier and friends took a tube to a mountain top and found a decrease in pressure. Now we know that the change of pressure with height can be described by hydrostatic equation. So using dp g , dz P gh (4.1) The hydrostatic balance can be applied to any fluids without motion. The mercury barometer's design gives rise to the expression of atmospheric pressure in inches or millimeters. A mercury barometer has a glass tube of at least 33 inches (about 84 cm) in height, closed at one end, with an open mercury-filled reservoir at the base. The pressure is quoted as the level of the mercury's height in the vertical column. 1 atmosphere is equivalent to about 29.9 inches, or 760 millimeters, of mercury. The use of this unit is still popular in the United States, although it has been disused in favor of SI or metric units in other parts of the world. Barometers of this type normally measure atmospheric pressures between 28 and 31 inches of mercury. So what does mercury barometer look like? Well a mercury barometer is shown below 2 P Hg g , z h , z (4.2) But both density and reading scale change with temperature. Gravity also changes with latitude and height. Thus, if one wants to convert a raw barometer reading P1 to the station pressure, temperature correction and gravity correction are needed. The station pressure Ps can be expressed as (4.3) P2 P1 CT (4.4) Ps P2 CG where CT and CG are the temperature and gravity corrections, respectively. The temperature correction CT may be written as (4.5) CT P1 ( )T where β and α are the volume expansion coefficient of mercury and scale, respectively. T is the barometer temperature. The units of T and P1 are determined based on the calibration of a specific barometer. Assuming a brass scale, the expansion coefficients are β=1.818 x 10-4 K-1 and α=1.840 x 10-5 K-1. The correction for local gravity is given by gL g0 (4.6) P2 g0 where g0=9.80665 m s-2 is the standard gravity. Note that the standard gravity is the reference value used in the barometry, it is not the value of gravity at latitude 45 0 at sea level. WMO suggests first to compute gravity at sea level at the barometer latitude Φ, CG g 9.80616(1 2.637310 3 cos(2) 5.9 10 6 cos2 (2)) and then do the height correction using g L g 3.08610 6 z 1.11810 6 ( z z ) where z is the barometer elevation in meters, and z´ is the mean elevation within a 150 km radius. (4.7) (4.8) Torricelli Barometers A glass tube is enclosed at one side. The tube is filled completely with mercury. Now all air was out of the tube. Locking the tube on the open side and turning the tube upside down in a reservoir filled with mercury. Now unlocking the opening, a certain amount of mercury will still kept in the tube. This is caused by the air pressure on the surface of the mercury in the reservoir pushed the mercury column up in the tube. This was the birth of the first actual and reliable weather instrument. Contra Barometers In the late 1690's in Holland, a "contra" barometer was developed, using a u-shaped multiple tube to measure air-pressure and used multiple types of liquids to display the air pressure (usually mercury and colored alcohol). They were called "contra" as the mercury level, due to design, decreased in height with an increase in air pressure, hence the name "contra". Contra barometer solved one of the major problems that 3 the Toricelli barometer showed: the degree of readability. In Contra barometer, a "multiplicator" is placed onto the mercury tube. In the U-shaped instrument, colored alcohol was filled onto the mercury surface. All changes in the mercury column where multiplied with a factor ten, and transferred to the surface of the colored alcohol. The scale of the Toricelli became tenfold enlarged, and easy to read due to the colored fluid. Fortin barometers Fortin barometers, sometimes referred to as weather service barometers, have been in use for over 100 years. They are probably the most accurate of all the barometer types. In the past, there was an instrument of this type in almost every science classroom in the country. They are still widely used in labs and weather stations. As of the spring of 2007, most of the companies have stopped producing any mercury instruments. Fortin barometers have a zero setting device, which can be used to adjust the level of the mercury in the cistern of the Fortin barometers. The main requirements of the place of exposure are uniform temperature, good light, solid and vertical mounting and protection against rough handling. Also great care should be taken when transporting a mercury barometer. All Fortin barometers are calibrated against standard instruments by complying with the recommendations of the WMO (World Meteorological Organisation). Eco-Celli Barometers The Eco-celli barometers are the newly developed liquid barometers. The Eco-celli contains no mercury. The U-shaped glass tube is filled with a red silicon-based fluid as well as a gas. The barometers scale is four times larger than a standard mercury barometer making it much easier to read the scale. The Eco-celli looks similar to a traditional mercury barometer but the basic principle is completely different, based on the compressibility of gasses instead of the weight of liquid mercury. On the upper left-hand side of the barometer, one can see the gas reservoir above the red fluid tube. The gas provides a constant counterpressure against the atmospheric pressure. Air pressure pushes down on the open side of the barometer tube onto the surface of the red fluid. As atmospheric pressure increases, the gas in the barometer will be compressed and you will notice the red fluid level in the right hand portion of the barometer tube falling. When the air pressure decreases, the gas in the barometer will expand and the red fluid will rise in the righthand portion of the barometer tube. A very important factor in measuring air pressure is the temperature. High temperatures will cause fluids and gases to expand causing an error in the readout of the barometer scale. The Eco-celli has solved that problem. On the right side of the Eco-celli is mounted a high precision thermometer parallel to the barometer tube. This thermometer has the same thermal expansion/compression rate as the barometer. With the help of a movable scale attached to both the barometer and thermometer tubes, you set the scale to the actual temperature (top of blue fluid level). The error caused by the temperature is automatically corrected and you can now read the correct air pressure. On the barometer scale, there is a slider. This is the tendency pointer. As you read the air pressure (top of red fluid) place the slider at that point. When taking a reading, sliding the entire scale up or down until the thermometer pointer is even with the top of the blue fluid level of the thermometer. By doing this, temperature correction is completed and pressure readings may be taken. Then, set the tendency pointer on the barometer tube to the top of the red fluid level and take your reading off of the scale. The next time you take a reading, you should see a difference between the location of the tendency pointer and the level of the red fluid. Unless there is severe weather approaching, air pressure does not change rapidly so allow several hours between readings The Eco-celli barometer is calibrated to sea level (0 feet). When you install the barometer in an area that has a different height (more than 100 feet) above or below sea level, you will need to calibrate your barometer. There are two methods of calibration. Method one: the first thing you will need is the current local air pressure. Contact a local airport, television station, National Weather Service office, or visit a weather web site such as weather.com. Once you have the correct local air pressure, remove fluid from the red tube using the calibration set until the fluid level matches the correct air pressure. Have paper towels 4 available to catch any spills as the colored oil will stain anything it comes in contact. The second calibration method is to calculate the air pressure difference from sea level. To find your height above sea level, contact your local city/town hall. Each 25 feet above sea level is equal to 0.03 inches on the scale. Remove the calculated amount of fluid until the correct fluid level is reached. Please save the removed fluid in the syringe so it may be added if the barometer is moved to a different location. Aneroid Barometers: Aneroid gauges are based on a metallic pressure sensing element which flexes elastically under the effect of a pressure difference across the element. "Aneroid" means "without fluid," and the term originally distinguished these gauges from the hydrostatic gauges described above. However, aneroid gauges can be used to measure the pressure of a liquid as well as a gas, and they are not the only type of gauge that can operate without fluid. For this reason, they are often called mechanical gauges in modern language. Aneroid gauges are not dependent on the type of gas being measured and are less likely to contaminate the system than hydrostatic gauges. An aneroid barometer uses a small, flexible metal box called an aneroid cell (or capsule). This aneroid capsule (cell) is made from an alloy of beryllium and copper. The evacuated capsule (or usually more capsules) is prevented from collapsing by a strong spring. Small changes in external air pressure cause the cell (or cells) to expand or contract. This expansion and contraction drives mechanical levers such that the tiny movements of the capsule are amplified and displayed on the face of the aneroid barometer. Many models include a manually set needle which is used to mark the current measurement so a change can be seen. A tube or diaphragm is evacuated to give a pressure sensitive element. Changes in the external pressure will cause a deflection that is proportional to the pressure change. These deflections can be used to give a mechanical reading or an electrical reading using resistance, capacitance, or strain gauge elements. Unlike the mercury barometer, sensor calibration is needed. Barograph A barograph is a recording aneroid barometer that records the barometric pressure over time. Barographs use one or more aneroid cells acting through a gear or lever train to drive a recording arm that has at its extreme end a pen, which records on paper using ink. The recording material (most of the time paper) is mounted on a cylindrical drum which is rotated slowly by clockwork. Commonly, the drum makes one revolution per day, per week, or per month and the rotation rate can often be selected by the user. The figure below shows an example of the barograph recording. Nowadays, mechanical recording barographs for meteorological use have commonly been superseded (though not all) by electronic weather instruments 5 that use computer methods to record the barometric pressure. These are not only less expensive than mechanical barographs but they may also offer both greater recording length and the ability to perform further data analysis on the captured data including automated use of the data to forecast the weather. Strain Gauge and the associated Electronic pressure sensors When external forces are applied to a stationary object, stress and strain are the result. Stress is defined as the object's internal resisting forces, and strain is defined as the displacement and deformation that occur. For a uniform distribution of internal resisting forces, stress can be calculated by dividing the force (F) applied by the unit area (A): F Stress A Strain is defined as the amount of deformation per unit length of an object when a load is applied. Strain is calculated by dividing the total deformation of the original length by the original length (L): L Strain L Typical values for strain are less than 0.005 inch/inch and are often expressed in micro-strain units: Micro Strain strain 106 Strain may be compressive or tensile and is typically measured by strain gages. It was Lord Kelvin who first reported in 1856 that metallic conductors subjected to mechanical strain exhibit a change in their electrical resistance. This phenomenon was first put to practical use in the 1930s. Fundamentally, all strain gauges are designed to convert mechanical motion into an electronic signal. A change in capacitance, inductance, or resistance is proportional to the strain experienced by the sensor. If a wire is held under tension, it gets slightly longer and its cross-sectional area is reduced. This changes its resistance (R) in proportion to the strain sensitivity (S) of the wire's resistance. When a strain is introduced, the strain sensitivity, which is also called the gauge factor (GF), is given by, R R R R GF L L Strain The ideal strain gauge would change resistance only due to the deformations of the surface to which the sensor is attached. However, in real applications, temperature, material properties, the adhesive that bonds the gauge to the surface, and the stability of the metal all affect the detected resistance. Because most materials do not have the same properties in all directions, knowledge of the axial strain alone is insufficient for a complete analysis. Other types of strains also need to be measured. Shearing strain considers the angular distortion of an object under stress. Imagine that a horizontal force is acting on the top right corner of a thick book on a table, forcing the book to become somewhat trapezoidal. 6 The shearing strain in this case can be expressed as the angular change in radians between the vertical yaxis and the new position. The shearing strain is the tangent of this angle. Bending strain, or moment strain, is calculated by determining the relationship between the force and the amount of bending which results from it. Although not as commonly detected as the other types of strain, torsional strain is measured when the strain produced by twisting is of interest. Sensor Designs (how to measure strains) The deformation of an object can be measured by mechanical, optical, acoustical, pneumatic, and electrical means. The earliest strain gauges were mechanical devices that measured strain by measuring the change in length and comparing it to the original length of the object. For example, the extension meter (extensiometer) uses a series of levers to amplify strain to a readable value. In general, however, mechanical devices tend to provide low resolutions, and are bulky and difficult to use. The most widely used characteristic that varies in proportion to strain is electrical resistance. Although capacitance and inductance-based strain gauges have been constructed, these devices' sensitivity to vibration, their mounting requirements, and circuit complexity have limited their application. The photoelectric gauge uses a light beam, and a photocell detector to generate an electrical current that is proportional to strain. The gauge length of these devices can be as short as 1/16 inch, but they are costly and delicate. Measuring Circuits In order to measure strain with a bonded resistance strain gage, it must be connected to an electric circuit that is capable of measuring the minute changes in resistance corresponding to strain. Strain gauge transducers usually employ four strain gauge elements electrically connected to form a Wheatstone bridge circuit (see figure below). A Wheatstone bridge is a divided bridge circuit used for the measurement of static or dynamic electrical resistance. The output voltage of the Wheatstone bridge is expressed in millivolts output per volt input. The Wheatstone circuit is also well suited for temperature compensation. In the figure below, if R1, R2, R3, and R4 are equal, and a voltage, VIN, is applied between points A and C, then the output between points B and D will show no potential difference. However, if R4 is changed to some value which does not equal R1, R2, and R3, the bridge will become unbalanced and a voltage will exist at the output terminals. In a so-called G-bridge configuration, the variable strain sensor has resistance Rg, while the other arms are fixed value resistors. Any small change in the resistance of the sensing grid will throw the bridge out of balance, making it suitable for the detection of strain. When the bridge is set up so that Rg is the only active strain gauge, a small change in Rg will result in an output voltage from the bridge. If the gauge factor is GF, the strain measurement is related to the change in Rg as, 7 Strain R g R g GF Ionization gauges Ionization gauges are the most sensitive gauges for very low pressures (also referred to as hard or high vacuum). They sense pressure indirectly by measuring the electrical ions produced when the gas is bombarded with electrons. Fewer ions will be produced by lower density gases. The ions are attracted to a suitably biased electrode known as the collector. The current in the collector is proportional to the rate of ionization, which is a function of the pressure in the system. Hence, measuring the collector current gives the gas pressure. The calibration of an ion gauge is unstable and dependent on the nature of the gases being measured, which is not always known. So, special calibration is needed. 8