Introduction
advertisement

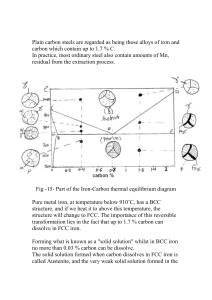
Subject: Engineering Materials and Metallurgy Course Objective: The objectives of this course are to: (1) reinforce fundamental concepts and introduce advanced topics in physical metallurgy, and (2) develop literacy in major alloy systems, with emphasis on microstructural evolution and structure-properties relations. From a foundation in modern physical metallurgy, the student will understand the basis for optimization of the structural metallic alloys that enable modern technology. Topics; including equilibrium phase diagrams, thermodynamics, diffusional and martensitic transformation kinetics, recrystallization, and grain growth; are discussed in conjunction with transition-metal alloys based on iron, nickel and titanium, as well as with thermomechanical processing methods. Historical Perspective Materials are so important in the development of civilization that we associate Ages with them. In the origin of human life on Earth, the Stone Age, people used only natural materials, like stone, clay, skins, and wood. When people found copper and how to make it harder by alloying, the Bronze Age started about 3000 BC. The use of iron and steel, a stronger material that gave advantage in wars started at about 1200 BC. The next big step was the discovery of a cheap process to make steel around 1850, which enabled the railroads and the building of the modern infrastructure of the industrial world. Materials Science and Engineering The combination of physics, chemistry, and the focus on the relationship between the properties of a material and its microstructure is the domain of Materials Science. The development of this science allowed designing materials and provided a knowledge base for the engineering applications (Materials Engineering). Advantages of Studying Materials Science and Engineering To be able to select a material for a given use based on considerations of cost and performance. To understand the limits of materials To change the material properties based on the use. To be able to create a new material that will have some desirable properties. UNIT-I Classification of Materials According to the way the atoms bound together: Metals: valence electrons are detached from atoms, and spread in an 'electron sea' that "glues" the ions together. Metals are usually strong, conduct electricity and heat well and are opaque to light (shiny if polished). Examples: aluminum, steel, brass, gold. Semiconductors: the bonding is covalent (electrons are shared between atoms). Their electrical properties depend extremely strongly on minute proportions of contaminants. They are opaque to visible light but transparent to the infrared. Examples: Si, Ge, GaAs. Ceramics: atoms behave mostly like either positive or negative ions, and are bound by Coulomb forces between them. They are usually combinations of metals or semiconductors with oxygen, nitrogen or carbon (oxides, nitrides, and carbides). Examples: glass, porcelain, many minerals. Polymers: are bound by covalent forces and also by weak van der Waals forces, and usually based on H, C and other non-metallic elements. They decompose at moderate temperatures (100 – 400 C), and are lightweight. Other properties vary greatly. Examples: plastics (nylon, Teflon, polyester) and rubber. Types of Bonding Ionic Bonding This is the bond when one of the atoms is negative (has an extra electron) and another is positive (has lost an electron). Then there is a strong, direct Coulomb attraction. An example is NaCl. In the molecule, there are more electrons around Cl, forming Cl- and less around Na, forming Na+. Ionic bonds are the strongest bonds. Covalent Bonding In covalent bonding, electrons are shared between the molecules, to saturate the valency. The simplest example is the H2 molecule, where the electrons spend more time in between the nuclei than outside, thus producing bonding. Metallic Bonding In the metallic bond encountered in pure metals and metallic alloys, the atoms contribute their outer-shell electrons to a generally shared electron cloud for the whole block of metal. Secondary Bonding (Van der Waals) Fluctuating Induced Dipole Bonds Polar Molecule-Induced Dipole Bonds Permanent Dipole Bonds Crystal Structures Atoms self-organize in crystals, most of the time. The crystalline lattice is a periodic array of the atoms. When the solid is not crystalline, it is called amorphous. Examples of crystalline solids are metals, diamond and other precious stones, ice, graphite. Examples of amorphous solids are glass, amorphous carbon (a-C), amorphous Si, most plastics Unit Cells The unit cell is the smallest structure that repeats itself by translation through the crystal. The most common types of unit cells are the faced-centered cubic (FCC), the body-centered cubic (FCC) and the hexagonal close-packed (HCP). Other types exist, particularly among minerals. Polymorphism and Allotropy Some materials may exist in more than one crystal structure, this is called polymorphism. If the material is an elemental solid, it is called allotropy. An example of allotropy is carbon, which can exist as diamond, graphite, and amorphous carbon. Polycrystalline Materials A solid can be composed of many crystalline grains, not aligned with each other. It is called polycrystalline. The grains can be more or less aligned with respect to each other. Where they meet is called a grain boundary. Imperfection in solids Materials are not stronger when they have defects. The study of defects is divided according to their dimension: 0D (zero dimension) – point defects: vacancies and interstitials Impurities. 1D – linear defects: dislocations (edge, screw, mixed) 2D – grain boundaries, surfaces. 3D – extended defects: pores, cracks Introduction to phase diagram Component: pure metal or compound (e.g., Cu, Zn in Cu-Zn alloy, sugar, water, in a syrup.) Solvent: host or major component in solution. Solute: dissolved, minor component in solution. System: set of possible alloys from same component (e.g., iron-carbon system.) Solubility Limit: Maximum solute concentration that can be dissolved at a given temperature. Phase: part with homogeneous physical and chemical characteristics Solid Solutions A solid solution occurs when we alloy two metals and they are completely soluble in each other. If a solid solution alloy is viewed under a microscope only one type of crystal can be seen just like a pure metal. Solid solution alloys have similar properties to pure metals but with greater strength but are not as good as electrical conductors. The common types of solid solutions are 1) Substitutional solid solution 2) interstitial solid solutions Substitutional solid solution : The name of this solid solution tells you exactly what happens as atoms of the parent metal ( or solvent metal) are replaced or substituted by atoms of the alloying metal (solute metal) In this case, the atoms of the two metals in the alloy, are of similar size. Interstitial solid solutions: In interstitial solid solutions the atoms of the parent or solvent metal are bigger than the atoms of the alloying or solute metal. In this case, the smaller atoms fit into interstices i.e spaces between the larger atoms. Phases One-phase systems are homogeneous. Systems with two or more phases are heterogeneous, or mixtures. This is the case of most metallic alloys, but also happens in ceramics and polymers. A two-component alloy is called binary. One with three components is called ternary. Microstructure The properties of an alloy do not depend only on concentration of the phases but how they are arranged structurally at the microscopy level. Thus, the microstructure is specified by the number of phases, their proportions, and their arrangement in space. A binary alloy may be a single solid solution two separated, essentially pure components. two separated solid solutions. a chemical compound, together with a solid solution. Phase diagram: A graph showing the phase or phases present for a given composition as a function of temperature. Polyphase material: A material in which two or more phases are present. Eutectoid: Transforming from a solid phase to two other solid phases upon cooling. Peritectoid: Transforming from two solid phases to a third solid phase upon cooling. Peritectoid reaction: A reaction in which a solid goes to a new solid plus a liquid on heating, and reverse occurs on cooling. Iron-Iron Carbon diagram is essential to understand the basic differences among iron alloys and control of properties Iron is allotropic; at room temperature pure iron exists in the Body Centered Cubic crystal form but on heating transforms to a Face Centered Cubic crystal. The temperature that this first transformation takes place is known as a critical point and it occurs at 910 degrees Celsius. This change in crystal structure is accompanied by shrinkage in volume, sine the atoms in the face centered crystal are more densely packed together than in the body centered cubic crystal. At the second critical point the F.C.C crystal changes back to a B.C.C crystal and this change occurs at 1390 degrees Celsius. Iron above 1390 degrees is known as delta iron Iron between 1390 and 910 degrees is known as gamma iron Iron below 910 degrees is known as alpha iron . Iron carbon diagram At 4.3% carbon composition, on cooling Liquid phase is converted in to two solids hence forming Eutectic reaction. L ↔ γ + Fe3C Eutectoid: 0.76 wt%C, 727 °C γ(0.76 wt% C) ↔ α (0.022 wt% C) + Fe3C Shown below is the steel part of the iron carbon diagram containing up to 2% Carbon. At the eutectoid point 0.83% Carbon, Austenite which is in a solid solution changes directly into a solid known as Pearlite which is a layered structure consisting of layers of Ferrite and Cementite UNIT II I. HEAT TREATMENT PROCESSES A. The process of heating a metal workpiece to a high temperature to change it's properties, normally to make the workpiece harder or softer. B. Basic heat treatment steps and equipment: 1. Heating to the correct temperature. a. Equipment normally a heat-treating furnace, a blowtorch, gas welding torch, or a forge. 2. Holding or soaking at this temperature for a certain length of time. a. Equipment can be the same furnace or forge. 3. Cooling in a way that will produce the desired results. a. Equipment can be container of water, tempering oil or brine. C. Heat treatment processes and uses. 1. Hardening a. The process: (1). Heating and cooling steel to increase its hardness and tensile strength, to reduce its ductility. (2). Requires a steel with a minimum of 0.20% carbon content. b. Uses: (1). To produce sharp-edged cutting tools. (2). To make bearing surfaces wear better/longer. (3). To put "spring" in a spring. 2. Tempering a. The process (1). Process of reducing the degree of hardness (removing brittleness) and strength and increasing toughness. (2). If a part is too hard, it will chip; if it is not hard enough, it will bend. Tempering achieves the balance between hardness and strength. b. Uses: (1). Knife blades, screw driver tip, cold chisel tip, axes, shears. 3. Annealing a. The process: (1). Process of softening metal to relieve internal strain and to make the metal easier to shape and cut. b. Uses: (1). Reusing old springs or files for other projects not requiring hardness. (2). To relieve built up stresses and prevent cracking of manufactured metals. II. HOT METAL FORMING PROCESSES A. Techniques to give objects shape or form without adding or removing any materials from the part. B. Metal forming operations. 1. Bending. a. The process: (1). Process by which metal is uniformly strained or stretched around a straight axis and results in a product having a linear or straight shape. b. Uses: (1). Decorative grillwork. 2. Forging (blacksmithing). a. The process: (1). Forming is achieved by hammering or applying steady pressure to a workpiece, forcing it to take the shape of a die. May be done with the workpiece either hot or cold. b. Uses: (1). Making small tools (chisels and center punches), horseshoes, ornamental ironwork. I. CLASSIFICATION AND USES OF METALS A. Ferrous metal types ("ferrous" = containing iron and alloys) 1.Iron. a. Rare in the pure state; pure iron is not used commercially. 2. Wrought iron. a. Contains: (1). Iron, alloyed (combined) with, (2). Less than 0.03% carbon. b. True wrought iron is scarce and expensive. c. True wrought iron forges well, can be easily bent hot or cold and can be welded. d. "Wrought iron" is currently used to refer to almost any malleable low carbon steel. 3. Carbon steels, or "steel". a. Contains: (1). Iron, alloyed (combined) with, (2). Carbon, (3). Less than 1.65% manganese, (4). Less than 0.60% copper, and (5). Smaller amounts of silicon, sulfur and phosphorous. b. Types: (1). Low-carbon ("mild") steels (a). Between 0.05% and 0.30% carbon. (b). Tough and ductile. Easily formed, machined and welded. (c). Most commonly used of the carbon steel types. (2). Medium-carbon steels (a). Between 0.30% and 0.45% carbon. (b). Strong and hard, but less ductile. (c). Not as easily welded, due to tendency to crack after welding. (d). Used for gears. (3). High-carbon steels (a). Between 0.45% and 0.75% carbon. (b). Very hard and strong, less ductile. (c). Special electrodes and welding procedures are required, to prevent brittleness and cracking. (d). Used for cold chisels and hammers. (4). Very-high-carbon steels (a). Between 0.75% and 1.5% carbon. (b). Super hard and strong. (c). Seldom welded; special electrodes and procedures used. (d). Used for tools and springs. (e). Can be used for items that must be hardened and tempered. (See paragraph IV. below for definition of these terms). 4. Rolled steels. a. Bar, rod and structural steels produced by rolling the steel into shape, much like an old clothes wringer. Cold rolled steel. Steel formed when cold. Results in more accurately sized, better surface finished product. Hot rolled steel. Metal formed into shape while the metal is red hot. Produces a uniform quality, commonly used steel. Bluish scale on the surface formed when water sprayed on the steel as it passes between rollers. 5. Galvanized steel. a. Mild steel coated with zinc to prevent rusting. b. Care should be taken not to inhale toxic fumes when welding this material. II. PROPERTIES OF METALS A. Tensile Strength. 1. Ability to resist being pulled apart in tension. Metal failures are often caused by forces exceeding the tensile strength of the part. B. Ductility. 1. Ability to be stretched or pulled through a die to form wire. 2. Copper is a very ductile metal. C. Hardness. 1. Ability to resist penetration. 2. Hardness can be increased by heat treatment or work hardening. III. IDENTIFICATION OF METALS A. Numbering system for carbon and alloy steels. 1. Four digit (sometimes five digits) numbering system to identify carbon and alloy steels: a. First digit usually indicates the principle element in the steel as follows: SERIES TYPES AND DESIGNATION CLASSES 10XX Non-resulferized carbon steel grades (plain carbon steel) 13xx Manganese 1.75% 20xx Nickel steels 23xx Nickel 3.5% 30xx Nickel-chromium steels* 31xx Nickel 1.25% - chromium 0.65 or 0.80% 40xx Molybdenum 0.25% 41xx Chromium 0.50 – 0.95% - molybdenum 0.15 or 0.20% 43xx Nickel 1.80% - chromium 0.50 or 0.80% - molybdenum 0.25%* 50xx Chromium 0.28 or 0.40% 51xx Chromium 0.80, 0.90, 0.95, 1.00 or 1.05% 5xxxx Carbon 1.00% - chromium 0.50, 1.00 or 1.45% 60xx Chrome-vanadium steels 61xx Chromium 0.80 or 0.95% - vanadium 0.10 or 0.15% min. 70xx Heat resisting casting alloys 80xx Nickel – chrome – molybdenum steels* 86xx Nickel 0.55% - chromium 0.50 or 0.65% - molybdenum 0.20% 90xx Silicon – manganese steels 92xx Manganese 0.85% - silicon 2.00% 93xx Nickel 3.25% - chromium 1.20% - molybdenum 0.12% *Stainless steels always have a high chromium content, often considerable amounts of nickel, and sometimes contain molybdenum and other elements. Stainless steels are identified by a three-digit number beginning with 2, 3, 4, or 5. b. Second digit (in alloy steels) represents the approximate percentage of alloy element. c. Third and fourth digits show the carbon content in points (where a point equals 100 times the percentage carbon). d. Examples: (1). 1095 steel is a carbon steel with 0.95% (95 points) carbon. (2). 2511 steel is nickel steel with approximately 5% nickel and 0.11% carbon. Austenite to Pearlite Transformation (a) Austenite-to-pearlite transformation of iron-carbon alloy as a functionof time and temperature. (b) Isothermal transformation diagram obtained from (a) for a transformation temperature of 675 °C (1247 °F). TTT Diagram CCT Diagram Phase Transformations and Production of Microconstituents takes TIME. Higher Temperature = Less Time. If you don’t hold at one temperature and allow time to change, you are “Continuously Cooling”. Therefore, a CCT diagram’s transition lines will be different than a TTT diagram. Tempering Martensite needs to be tempered to get better ductility. This happens when Fe3C is allowed to precipitate from the supercooled Martensite. Process Annealing — Eliminating Cold Work: A low-temperature heat treatment used to eliminate all or part of the effect of cold working in steels. Annealing and Normalizing — Dispersion Strengthening: Annealing - A heat treatment used to produce a soft, coarse pearlite in steel by austenitizing, then furnace cooling. Normalizing - A simple heat treatment obtained by austenitizing and air cooling to produce a fine pearlitic structure. Spheroidizing — Improving Machinability: Spheroidite - A microconstituent containing coarse spheroidal cementite particles in a matrix of ferrite, permitting excellent machining characteristics in high-carbon steels. HOT METAL WORKING TECHNIQUES A. Holding stock. 1. Select tongs to fit the work. Jaws should be parallel when clamped on the stock. If necessary, heat the jaws to a cherry red and bend them to fit and hold the stock securely. 2. Keep the tongs cool by dipping them frequently in water. B. Heating stock. 1. Forge. a. Most economical for heating large pieces of metal. b. Place the stock in the fire in a horizontal position so that the surface of the metal will be heated uniformly. c. Observe the piece periodically to ascertain that it is heating uniformly (i.e., look at its color). 2. Oxy-acetylene torch flame. a. Best for smaller pieces. b. Move the flame over the entire surface to be worked to prevent overheating in spots and to provide sufficient heat to last until the working operation is completed. C. Hardening stock. 1. Requires carbon steel with greater than 0.20% carbon, i.e. steels at least at the high end percentage carbon of lo carbon steels. 2. Heat the steel to the critical temperature for the type of steel to be hardened. a. The color of the glow of the metal is a rough indicator of the temperature: Oxides on clean surface: yellow 430.°F straw 470. brown 500. purple 540. blue 560. Glow of metal: black red 875.°F dark red 975. cherry red 1450. critical temperature for mild steel yellow 2000. white 2300. sparks 2550. drips 2800. b. Carbon steel becomes nonmagnetic at the critical temperature and could also be an indicator.or, c. A thermometer in the furnace is the best method. 3. Remove the piece to be hardened and plunge it into the cooling solution and move it about rapidly to cool the metal quickly and evenly. Do not drop the piece into the coolant as it will not cool rapidly enough. a. Fresh water is cheapest. b. Brine (5 to 10% salt) in water. c. Mineral oil allows for slower quenching and tends to decrease the chances of cracking and warping. 4. Check the piece for hardness: a. A new file run across the edge of the piece will not cut in or take hold. D. Tempering - process of reducing the degree of hardness (removing brittleness) and strength and increasing toughness. 1. Complete the hardening process as described above (remember that the hardening process will not work on carbon steel with less than 0.20% carbon). 2. Thoroughly clean (use wire wheel and/or emery paper) off the carbon from the surface of the metal in the location where the piece is to be tempered. The metal should be "shiny" clean in order to view the surface colors during the following tempering process. 3. Reheat the tool (or portion) of the tool to be tempered to the appropriate tempering temperature. Note: the color will appear on the surface of the metal part. Note: the higher the tempering temperature used, the more hardness removed Tempering temperatures are normally between 430 and 530 degrees Fahrenheit. TEMPERING TEMPERING TEMPERATURE IN TOOL COLOR DEGREES FARENHEIT HAMMER FACE, PALE YELLOW 430 TO 450ºF SCRAPER CENTER PUNCH, FULL YELLOW 470ºF DRILL SCREWDRIVER, STRAW BROWN 490 TO 510ºF COLD CHISEL 4. Quench that portion of the tool to be tempered. or, 5. To simultaneously harden and temper a part (cold chisel for example): a. Heat the piece of metal to a cherry red color (hardening color). b. Hold the piece vertically and dip the end to be tempered (tip end of the screwdriver) and rapidly move the tip into and around in the water (or oil). This will harden the working end. c. When the tip appears cool, remove the screwdriver tip from the water (or oil) and brighten the tip with a piece of emery cloth. d. Observe the tip end and you will notice colors gradually move down from the hot portion to the tip end. (1). You will first notice a light straw color then, (2). a dark straw color, then, (3). a light brown color, then, (4) a dark brown color. e. When a dark brown color appears at the tip end, plunge the tip end into the water (or oil) and the tempering process will be achieved. 29 E. Annealing - the process of softening metal to relieve internal strain and to make the metal easier to shape and cut. 1. Heat the piece to the critical temperature (approximately 1450º F. for mild steel, i.e., a cherry red color). 2. Allow the piece to cool slowly (in the shut-down furnace, packed in sand, clamped between two pieces of hot metal or in a pile of fireplace bricks). The effect of carbon and heat treatment on the properties of plain-carbon steels. (a) White cast iron prior to heat treatment ( 100). (b) Ferritic malleable iron with graphite nodules and small MnS inclusions in a ferrite matrix ( 200). (c) Pearlitic malleable iron drawn to produce a tempered martensite matrix ( 500). (Images (b) and (c) are from Metals Handbook, Vols. 7 and 8, (1972, 1973), ASM International, Materials Park, OH 44073.) (d) Annealed ductile iron with a ferrite matrix ( 250). (e) As-cast ductile iron with a matrix of ferrite (white) and pearlite ( 250). (f) Normalized ductile iron with a pearlite matrix ( 250). Isothermal Heat Treatments Austempering - The isothermal heat treatment by which austenite transforms to bainite. Isothermal annealing - Heat treatment of a steel by austenitizing, cooling rapidly to a temperature between the A1 and the nose of the TTT curve, and holding until the austenite transforms to pearlite. Application of Hardenability Jominy test - The test used to evaluate hardenability. An austenitized steel bar is quenched at one end only, thus producing a range of cooling rates along the bar. Hardenability curves - Graphs showing the effect of the cooling rate on the hardness of as-quenched steel. Jominy distance - The distance from the quenched end of a Jominy bar. The Jominy distance is related to the cooling rate. The setup for the Jominy test used for determining the hardenability of steel Quench and Temper Heat Treatments Retained austenite - Austenite that is unable to transform into martensite during quenching because of the volume expansion associated with the reaction. Tempered martensite - The microconstituent of ferrite and cementite formed when martensite is tempered. Quench cracks - Cracks that form at the surface of a steel during quenching due to tensile residual stresses that are produced because of the volume change that accompanies the austenite-to-martensite transformation. Marquenching - Quenching austenite to a temperature just above the MS and holding until the temperature is equalized throughout the steel before further cooling to produce martensite. The effect of tempering temperature on the mechanical properties of a 1050 steel. Increasing carbon reduces the Ms and Mf temperatures in plain-carbon steels. Effect of Alloying Elements Hardenability - Alloy steels have high hardenability. Effect on the Phase Stability - When alloying elements are added to steel, the binary Fe-Fe3C stability is affected and the phase diagram is altered. Shape of the TTT Diagram - Ausforming is a thermomechanical heat treatment in which austenite is plastically deformed below the A1 temperature, then permitted to transform to bainite or martensite. Tempering - Alloying elements reduce the rate of tempering compared with that of a plain-carbon steel. Surface Treatments Selectively Heating the Surface - Rapidly heat the surface of a mediumcarbon steel above the A3 temperature and then quench the steel. Case depth - The depth below the surface of a steel at which hardening occurs by surface hardening and carburizing processes. Carburizing - A group of surface-hardening techniques by which carbon diffuses into steel. Cyaniding - Hardening the surface of steel with carbon and nitrogen obtained from a bath of liquid cyanide solution. Carbonitriding - Hardening the surface of steel with carbon and nitrogen obtained from a special gas atmosphere. (a) Surface hardening by localized heating. (b) Only the surface heats above the A1 temperature and is quenched to martensite. Outline of Heat Treatment Processes for Surface Hardening Proces Metals Elemen Procedure General Typical s harden t added Characteristic applicatio ed to s ns surface Carburiz Lowing C Heat steel at 870– A hard, high- Gears, carbon 950 °C (1600– carbon surface cams, steel 1750 °F) in an is (0.2% atmosphere C), alloy carbonaceous steels gases (0.08– carburizing) 0.2% C) carbon-containing < solids 0.060 produced. shafts, of Hardness to 65 55 bearings, HRC. piston pins, (gas Case depth < sprockets, or 0.5–1.5 mm ( clutch 0.020 to plates in.). (pack carburizing). Some Then quench. distortion part of during heat treatment. Carbonit Lowriding C and N Heat steel at 700– Surface °C Bolts, nuts, carbon 800 (1300– hardness 55 to gears steel 1600 °F) in an 62 HRC. Case atmosphere of depth 0.07 to carbonaceous gas 0.5 mm (0.003 and ammonia. to 0.020 in.). Then quench in Less distortion oil. than in carburizing. Cyanidi Low- C and N Heat steel at 760– Surface ng carbon 845 steel 1550 (0.2% molten C), alloy solutions steels cyanide (e.g., 30% (0.001 (0.08– sodium 0.2% C) and other salts. °C Bolts, nuts, (1400– hardness up to screws, °F) in bath a 65 HRC. Case small gears of depth 0.025 to of 0.25 mm to cyanide) 0.010 in.). Some distortion. Nitriding Steels N Heat steel at 500– Surface Gears, (1% Al, 600 °C (925–1100 hardness up to shafts, 1.5% °F) Cr, atmosphere 0.3% ammonia gas or 0.1 to 0.6 mm cutters, Mo), mixtures of molten (0.005 alloy cyanide salts. No 0.030 in.) and bars, steels further treatment. in an 1100 of Case HV. sprockets, depth valves, to boring fuel- 0.02 to 0.07 injection (Cr, mm (0.001 pump parts Mo), to 0.003 in.) stainles for high speed s steels, steel. highspeed tool steels Boronizi Steels B ng Part is heated Extremely using Tool and boron- hard and wear die steels containing gas or resistant solid in contact surface. Case with part. depth 0.025– 0.075 mm (0.001– 0.003 in.). Flame Medium None Surface is heated Surface Gear and hardeni -carbon with ng steels, oxyacetylene cast torch, then depth 0.7 to 6 axles, irons quenched with mm (0.030 to crankshafts an hardness 50 to sprocket 60 HRC. Case teeth, water spray or 0.25 in.). Little , other quenching distortion. methods. piston rods, lathe beds and centers Inductio Same n hardeni ng None Metal part is Same as Same as placed in copper above above above induction coils and is heated by high frequency current, then quenched. as Induction Heating Types of coils used in induction heating of various surfaces of parts UNIT-III The common alloying elements added to steel are Aluminium Boron Carbon Chromium Cobalt Copper Manganese Molybdeum Nickel Silicon Tungsten Vanadium Alloy steel are the types of steel in which elements other than carbon an iron are present in sufficient quantities to modify the properties of the materials. Alloying elements added for the purpose are: To increase hardenability To improve strength To improve mechanical properties To increase wear resistance To increase corrosion resistance To improve magnetic properties Classification of alloy steel Low alloy steel High alloy steel Alloy steel can also be subdivided as follows: Tool steel Stainless steel Maraging steel HSLA steel Copper and its alloys Copper has a density of 8900Kg/m3 and melting point of 1083oc. The properties of copper are: High electrical conductivity High thermal conductivity Good corrosion resistance Non-magnetic In addition, copper can be subjected to welding, brazing and soldering. Copper alloys: Brass Bronze Copper nickel alloys Precipitation harden able alloys Aluminum and its alloys are: Commercial aluminium Cast aluminium Wrought aluminium alloy UNIT-IV Polymers are materials of complex structure. Polymer consists of many units. In polymer, molecules of ordinary sizes are combined in long chains. Types of polymers Plastics Fibers Elastomers Polymers with fillers, solvents, plasticers and pigments are known as plastic. Types of plastics: Thermo plastic Thermoset plastic Major Characteristics of plastics are: Non crystalline structure Low softening temperature Resistance to chemical reaction Visco- elastic behaviour Non-conductors Low thermal conductivity Engineering thermoplastics are: Polythene Polyvinylchloride Polypropene Polystyrene Polymethyl Methacrylate Polyesters Polycarbonates Polyamides ABS UNIT-V When material is stressed below its elastic limit, the strain formed is temporary. When a material is stressed above its elastic limit, the plastic deformation takes place. Plastic deformation may takes place by: Slip Twinning Types of Mechanical tests: Tensile test Compressive test Shear test Hardness test Fatigue test Creep test Impact test