File - STEP in STEM
advertisement
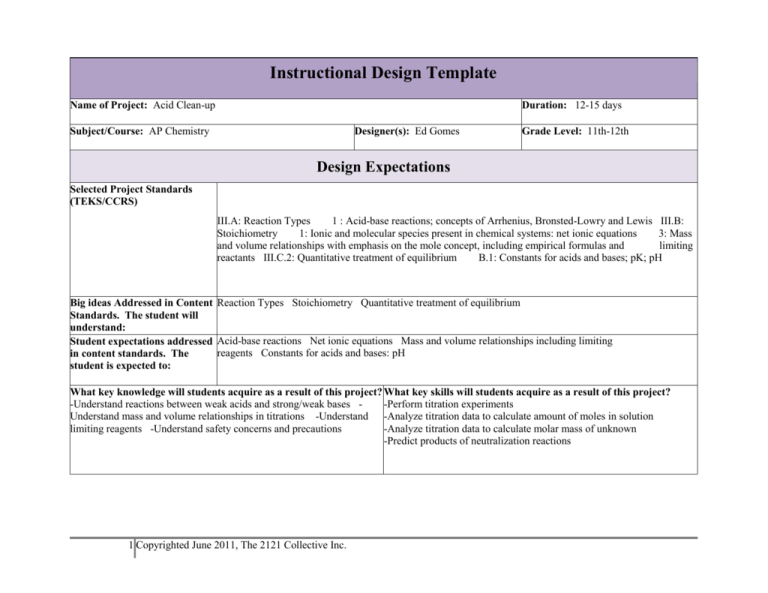
Instructional Design Template Name of Project: Acid Clean-up Duration: 12-15 days Subject/Course: AP Chemistry Designer(s): Ed Gomes Grade Level: 11th-12th Design Expectations Selected Project Standards (TEKS/CCRS) III.A: Reaction Types 1 : Acid-base reactions; concepts of Arrhenius, Bronsted-Lowry and Lewis III.B: Stoichiometry 1: Ionic and molecular species present in chemical systems: net ionic equations 3: Mass and volume relationships with emphasis on the mole concept, including empirical formulas and limiting reactants III.C.2: Quantitative treatment of equilibrium B.1: Constants for acids and bases; pK; pH Big ideas Addressed in Content Reaction Types Stoichiometry Quantitative treatment of equilibrium Standards. The student will understand: Student expectations addressed Acid-base reactions Net ionic equations Mass and volume relationships including limiting reagents Constants for acids and bases: pH in content standards. The student is expected to: What key knowledge will students acquire as a result of this project? What key skills will students acquire as a result of this project? -Understand reactions between weak acids and strong/weak bases -Perform titration experiments Understand mass and volume relationships in titrations -Understand -Analyze titration data to calculate amount of moles in solution limiting reagents -Understand safety concerns and precautions -Analyze titration data to calculate molar mass of unknown -Predict products of neutralization reactions 1 Copyrighted June 2011, The 2121 Collective Inc. How will students demonstrate they have acquired this knowledge? -Correctly predict products of neutralization rxns through net ionic equations -Select appropriate strong and weak bases that will correctly carry out a desired neutralization reaction -Correctly perform titration experiments and calculations -Write a report proposing safety procedures based on their selection of bases, including justification for their choices How will students demonstrate they have acquired these skills? -Correctly predict products of neutralization rxns by writing net ionic equations -Select appropriate strong and weak bases that will correctly carry out a desired neutralization reaction -Correctly perform titration experiments and calculations -Write a report proposing safety procedures based on their selection of bases, including justification for their choices Guiding questions to frame the inquiry and lead students to understanding: -What acid was spilled and what was the concentration (molarity)? -How much baking soda was truly needed to neutralize the spill (limiting/excess reagent)? -What is an efficient and proven method to neutralize accidental acid spills? Specific PBL Considerations A chemical spill of an unknown weak acid occurred at a middle school in our district. A substitute teacher handled the spill inappropriately and a student took a picture with his cell phone. The picture has leaked to the media and the district Project Idea: is under public scrutiny. The Board of Trustees needs AP Chemistry students to identify the spilled acid and its molarity, Summary of the issue, calculate the amount of baking soda that was truly needed to neutralize it, and predict the product(s) of the neutralization problem, or challenge reactions. Students need to then develop a method of neutralizing acids in such a way that teachers, substitutes, and students just as effectively. 2 Copyrighted June 2011, The 2121 Collective Inc. Launch Details Entry Document Details: Show students video regarding major acid spill that Authentic Format (ex. RFP) occurred near central China in June 7, 2011. Discuss Formal letter from district’s board of trustees the importance of spill analyses and safety evaluation. STEM Connections Acid-base chemistry Stoichiometry/Dimensional Analysis Use of digital technology to collect, analyze, and report data Development of safety methods Specific references to standards (ill defined) Acid-base chemistry; stoichiometry Entry Launch and Document Culminating Product Guidelines -Each lab report written in AP chem. format (including data and calculations) to be released on student created website/blog according to teacher’s schedule (format available in resources section) Content Constraints -The analysis of the acid spill is a convergent process. There is only one unknown and molarity, and the goal is to apply titration chemistry appropriately to find the right answer. -The development of safety methods is a divergent process with several possible solutions. However, two key ideas should be present: (1) the neutralizing agent should be a weak base (2) that is used as an excess reagent. Time constraints 12-15 days Material Constraints 4 weak acids Titration lab materials Vernier probes and TI nspire-calculator 3 Copyrighted June 2011, The 2121 Collective Inc. Facilitation Questions: KNOW 1. Writing net ionic equations and predicting reaction products for other types of reactions 2. Understand acid structure and pH calculations Know/Need to Know Activity NEED TO KNOW BASED ON LAUNCH EVENT 1. How did the Chinese government determine the acidity level in the water? 2. What do you think should be done after the clean-up is completed? Learning Experiences Description: Students will create a blog or website (e.g.: wordpress or goggle sites) where they post each piece of the puzzle in the analyses of the acid spill as they work through the project and their final recommendation for district training. Culminating Product/Assessment Connections to Standards: In doing so, the students will demonstrate their mastery of all of the following: Acid-base reactions Net ionic equations Mass and volume relationships including limiting reagents Constants for acids and bases: pH Essential Content Questions Interpret shape of titration curves Calculate the unknown molarity of an acid/base through titration calculations Determine the molar mass of an unknown weak diprotic acid through titration Write net ionic equations for neutralization rxns Write safety standards based on understanding of stoichiometry and acid-base chemistry Activity Sequence to Scaffold Content Development 4 Copyrighted June 2011, The 2121 Collective Inc. Activity/Workshop -Acid-base reactions lab -Post-lab includes prediction of the products that were made based on observation and net ionic equations (e.g., HCl + NaOH produce a white solid after evaporation… It must be NaCl) Academic Purpose/Outcome -Allow students to experiment neutralization rxns and other acid/bases, etc Content Rubric/Checklist Elements -Correctly write net ionic equations for rxns b/t strong/weak acids/bases; acids/bases + metals Formative Assessment: Content Elements See left Strategies Have students write their own predicted net ionic equations for the rxns in the lab. Then teacher can go over them as a class. Practice problems (e.g., homework) with names of reactants. Students must write net ionic equations predicting the products on their own. Differentiation considerations: Students can start working on practice problems/homework in class in groups. Teacher can then use this time to help students who are having the greatest struggle. The goal is to allow time for each student to master this concept individually. If time allows, teacher can demo a titration before next class so the students can be ready for next class. This activity can be spread over two days. 5 Copyrighted June 2011, The 2121 Collective Inc. Activity/Workshop Academic Purpose/Outcome -Titration Curves Lab (Chemistry Familiarize with shapes of Vernier #23) and post-lab titration curves, endpoints, and calculations choice of indicator -When done, do the same for their unknown Content Rubric/Checklist Elements Formative Assessment: Content Indicators -Differentiate between See left mono/diprotic acid/base -Choose appropriate indicator for Strategies Go over the Vernier lab either as their unknown -Differentiate titration curves of a class or groups for a formative assessment. Allow students to strong/weak acids/bases and then repeat the lab with their mono/diprotic acids unknown and use that as a summative assessment. Differentiation considerations: Students who complete the lab and conclusions successfully can start the analyses for their unknown immediately. Teacher can then work with students who need practical help on using equipment and/or technology. Heterogeneous grouping will allow students to help each other as they work through this project. Up to 2 days for this activity. Activity/Workshop Academic Purpose/Outcome -Acid-Base Comp. Lab (Adv. Chem Combination of acid-base rxns and Vernier #7) and post-lab stoichiometry calculations -After completed, students do the same procedures for their unknown Content Rubric/Checklist Elements Correctly calculate the unknown molarities Formative Assessment: Content Elements See left Strategies Vernier lab write-up includes postlab questions/calculations. Students work as a group and teacher can go over calculations as a class/or groups. Students do calculations for their unknown as summative. Differentiation considerations: Some students will struggle with stoichiometry calculations, especially for the diprotic unknowns. Heterogeneous grouping might allow students to help each other as they work through this project. Up to 2 days for this activity. 6 Copyrighted June 2011, The 2121 Collective Inc. Activity/Workshop -Titration of Diprotic Acid (Vernier #25) (determination of molar mass) and post-lab calculations Academic Purpose/Outcome Content Rubric/Checklist -Higher level evaluation and Elements synthesis of stoichiometry and acid- -Correctly calculate the molar mass base rxns concepts of diprotic unknown -Error analysis -Error analysis Formative Assessment: Content Elements See left Strategies Again, use the Vernier lab as a formative assessment where students work in groups first and teacher goes over information when all are done or individually in groups. Error analysis in post-lab calculations is important. Later, students complete lab as part of project/summative assessment. Differentiation considerations: Vernier lab can be reviewed individually, groups, or whole class. Up to 2 days for this activity. Teacher may choose to do a known diprotic acid with the students and then later allow them to complete the lab with the unknown. The teacher might also allow the students to work directly on the unknown as summative work. They’ll have done a lot of titrations by now and will have a lot of experience with the equipment and calculations. Depending on the level of different students/groups, the teacher can allow some to go straight to working with their unknown while others do a practice run with the teacher first. 7 Copyrighted June 2011, The 2121 Collective Inc. Activity/Workshop Academic Purpose/Outcome Calculate amount of baking soda in Stoichiometry analysis in grams grams that was necessary to (not just molarity). neutralize spill. Content Rubric/Checklist Elements Correctly compute amount of baking soda necessary in grams. Formative Assessment: Content Elements See left. Strategies At this point, students have had extensive practice in stoichiometry. Teacher should give some feedback in setting up problems with set up but not calculations. Calculations are summative. Differentiation considerations: Ideally, at this point, AP Chemistry students have mastered stoichiometric calculations. Some will do this very quickly. Students who struggle should work in a group and depend on teacher minimally. Teacher should give feedback on set up but allow students to work mostly independently. Ideally, 30 minutes max should be devoted to this. Activity/Workshop Develop clean up method. Academic Purpose/Outcome Content Rubric/Checklist Formative Assessment: Demonstrate understanding of acid- Elements Content Elements base neutralization rxns (weak v. -Selected bases should, in most See left strong) and stoichiometry (what cases, be weak and in the solid state should be the limiting reagent). -Student should conclude that the Strategies base must be the excess reagent -Check with students periodically -There are many possible choices of (informal assessment). Ask them bases that would be appropriate whether the neutralizing base should be (1) a strong/weak base and (2) limiting/excess reagent. -Schedule some time for peer assessment with student from a different group then the group they’ve been working with 8 Copyrighted June 2011, The 2121 Collective Inc. Differentiation considerations: If students are struggling with coming up with the procedures, give them guiding questions: (1) should you select strong or weak bases? Why? (2) What must be the excess reagent, the acid or the base? (3) Pick some bases that meet your criteria for #1. (4) Write your safety method. 1-1.5 days. Continue here if additional activities/experiences will be used: If desired, the teacher can continue the project throughout an acid-base equilibrium unit. Students can calculate pKa’s for the two protons in their diprotic unknown and complete error analysis. Other Planning Considerations 9 Copyrighted June 2011, The 2121 Collective Inc. Resources Launch event video Entry document Acid-base rxns lab: some ideas below Strong acid + strong base Strong acid + weak base Weak acid + strong base Weak acid + weak base Metal + acid Other rxns. Expose students to as many as possible. Products should be analyzed qualitatively and quantitatively to help students predict the products. Some examples follow below. Teacher can choose reactions based on what s/he has available in a chemical storage. Zn + HCl H2 + ZnCl2 Hydrogen gas that is released can be trapped and “lit” with a wooden splint. Students should connect flammable gas with hydrogen (if they know that information). HCl + NaOH H2O + NaCl Place in evaporating dish and allow to boil. White solid will be left. See if students can predict the product is table salt based on the formulas of reactants. Or if rxn is done carefully, if the solution’s pH after rxn is done should be near pH 7. That will lead students to predict that one of the products is H2O. Vernier Labs Vernier Probes TI-nspire calculator can be used for data collection from the Vernier probes. Lab rubric Student checklist: Can be used as a rubric if teacher adds points. 10 Copyrighted June 2011, The 2121 Collective Inc. Other None of the learning experiences above include the Launch Event, Entry Document, or Know/Need to Know activity. These are very important to engage students and to help them internalize the material learned. Required prior knowledge: Basic understanding of acid-base theory (Arrhenius, Bronsted-Lowry, Lewis) Distinguish strength of acids based on dissociation in water Writing net ionic equations (for other types of reactions, such as precipitation rxns) Stoichiometry and limiting reagents (this project allows students to apply topics learned in stoichiometry unit while learning about acidbase rxns and titrations) I suggest giving the following information to students with the entry letter, or soon thereafter. The letter does not give sufficient information for the project to be completed. It might be of benefit to see if any of the topics mentioned below show up in the Know/Need to Know activity. Also the material below provides necessary constraints for the students. A package of four weak diprotic acids arrived at a science teacher when a substitute was present. The box fell and one of the bottles spilled. Unfortunately, the chemical labels had been inappropriately labeled and were smudged. In desperation, the substitute asked one of the 7th graders what to do and was advised by one student to pour baking soda on the spilled acid. The substitute teacher poured 500g of baking soda onto the acid. The clean-up ended up being very messy. Out of desire to help the substitute teacher, several students ended up touching the mixture. A student took a picture with his cell phone and forwarded it to his parents. As you know, the picture has made its way to the school board and the media. All solutions were in 1L bottles. The bottle that spilled did not lose all the acid. 351mL was retained and we have shipped it to you with this letter (this letter is the entry document). Here is the list of acids that the science teacher had ordered. Most of these acids are weak acids that are commonly found in food. 1. Oxalic acid 2. Tartaric acid 3. Malic acid 4. Ascorbic acid The spill analysis section of this project is purely convergent and matches the style of the AP exam. The safety development is divergent and allows the student to apply qualitative principles of stoichiometry and acid-base neutralization reactions to a problem with several possible efficient solutions. 11 Copyrighted June 2011, The 2121 Collective Inc. 12 Copyrighted June 2011, The 2121 Collective Inc.








