File
advertisement

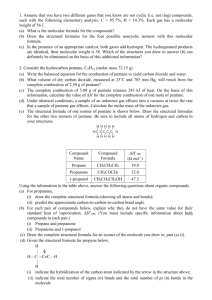
Name __________________________________________________________ Per ______ AP CHEMISTRY SEMESTER 1 EXAM FREE RESPONSE Question 1: Suppose that a stable element with atomic number 119, symbol Q, has been discovered. (a) Write the ground-state electron configuration for Q, showing only the valence shell electrons. (b) Would Q be a metal or a nonmetal? Explain in terms of electron configuration. (c) On the basis of periodic trends, would Q have the largest atomic radius in its group or would it have the smallest? Explain in terms of electronic structure. (d) What would be the most likely charge of the Q ion in stable ionic compounds? Question 2: For each of the following three reactions, in part (i) write a balanced question for the reaction and in part (ii) answer the question about the reaction. In part (i), coefficients should be in terms of the lowest whole numbers. Assume that solutions are aqueous unless otherwise indicated. Represent substances in solutions as ions if the substances are extensively ionized. Omit formulas for any ions or molecules that are unchanged by the reaction. You may use the empty space on scratch paper but only equations that are written in the answer boxes provided will be graded. (a) A solution of sodium hydroxide is added to a solution of lead (II) nitrate. (i) Balanced equation: (ii) If 1.0 L volumes of 1.0 M solutions of sodium hydroxide and lead (II) nitrate are mixed together, how many moles of product(s) will be produced? Assume the reaction goes to completion. (b) Excess nitric acid is added to solid calcium carbonate. Balanced equation: (c) A solution containing silver (I) ion (an oxidizing agent) is mixed with a solution containing iron (II) ion (a reduction agent). (i) Balanced equation: The species represented below all have the same number of chlorine atoms attached to the central atom. Question 3 (a) Draw the Lewis structure (electron dot diagram) of each of the four species. Show all valence electrons in your structures. GeCl4 SeCl4 ICl4- ICl4+ (b) On the basis of the Lewis structures drawn in part (a), answer the following questions about the particular species indicated. (i) What is the Cl – Ge – Cl bond angle in GeCl4? (ii) Is SeCl4 polar? Explain. (iii) What is the hybridization of the I atom in ICl4-? (iv) What is the geometric shape formed by the atoms in ICl4+? Question 4: Consider hydrocarbon pentane, C5H12 (molar mass 72.15 g). (a) Write the balance equation for the combustion of pentane to yield carbon dioxide and water. (b) If only 5.00 g of oxygen is available to combust 2.50 g of pentane. Which is the limiting reactant? (c) How many grams of the reactant that is in excess is left over? Question 5: Answer the following questions about thermodynamics. Substance Combustion Reaction Enthalpy of Combustion, ΔHocomb, at 298 K (kJ mol-1) H2(g) H2(g) + ½ O2(g) H2O(l) -290 C(s) C(s) + O2(g) CO2(g) -390 CH3OH(l) -730 a) In the empty box in the table above, write a balanced chemical equation for the complete combustion of one mole of CH3OH(l). Assume products are in the standard states at 298 K. Coefficients do not need to be whole numbers. b) On the basis of your answer to part a) and the information in the table, determine the enthalpy change for the reaction C(s) + H2(g) + H2O(l) CH3OH(l). Question 6: A 1.50 L container holds a 9.62 g sample of an unknown gaseous saturated hydrocarbon at 30 degrees C and 3.62 atm. i) Calculate the density of the gas. ii) Calculate the molar mass of the gas. iii) Write the formula of the hydrocarbon.