Worksheet Package 1,2,3
advertisement

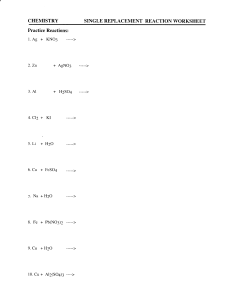
CHEMISTRY 11 EQUATION WORKSHEET #1 A. BALANCING EQUATIONS FOR SYNTHESIS REACTIONS. Synthesis or combination reaction involves the combination of two or more substances to form a compound. Balance the following equations by writing the simplest whole number coefficient in the space provided. 1. ____ H2 (g) + ______ F2 (g) ----- > _____ HF(g) 2. _____ Sb(s) + _____ Br2(l) -------- > ________ SbBr3(g) 3. ______ Ag + ______ O2 ---->______ Ag2O 4. ______ H2(g) + ______ Cl2(g) ---->______ HCl(g) 5. _____ Na(s) + _____ O2(g) ------ > ______ NaO(s) Write in the formulae for the products in the following synthesis reactions and then balance the equations. - To predict the product, you must join the two reactants together. - The formula of the product is determined by examining the combining capacities of each reactant. Do not simply join the two together maintaining the same coefficient of the reactants. 6. ____ Ca + O2 ------ > _____ ________ 7. ____ Al + ____ S8 ------ > _____ _______ 8. _____ Al + _____ O2 ------ > ______ ________ 9. _____ Fe + _____ I2 ----- > ______ ______ 10. _____ Zn + _____ O2 ----- > ____ ________ ( assume iron forms a ferric ion) CHEMISTRY 11 EQUATION WORKSHEET #2 B. BALANCING EQUATIONS FOR SIMPLE DECOMPOSITION REACTIONS. Decomposition reaction involves breaking down a molecule into simpler substances. Balance the following equations by writing in the simplest whole number coefficients in the spaces provided. 1. ______ NaCl ----> ______ Na + ______ Cl2 2. ______ CaBr2 ----> ______ Ca + ______ Br2 3. ______ CCl4 ----> ______ C + ______ Cl2 4. ______ NCl3 ----> ______ N2 + ______ Cl2 5. ______ P4O10 ----> ______ P4 + ______ O2 Write in the formulae for the products in the following simple decomposition reactions and then balance the equations. 6. ______ KI ----> ____ ______ + _____ ______ 7. ______ AlCl3 ----> ____ ______ + ____ _______ 8. ______ CuO ----> ____ _______ + ____ _______ 11. ______ NF3 ----> ______ ________ + ______ ________ 12. ______ NiO ----> ______ ________ + ______ ________ 13. ______ LiCl ----> ______ ________ + ______ ________ 14. ______ H2S ----> ______ ________ + ______ ________ 15. ______ HgO ----> ______ ________ + ______ ________ CHEMISTRY 11 EQUATION WORKSHEET 3 C. BALANCING EQUATIONS FOR SINGLE REPLACEMENT REACTIONS Balance the following equations by writing in the simplest whole number coefficients in the spaces provided. 1. ______ Fe + ______ CuCl2 ---> ______ Cu +______ FeCl2 2. ______ Zn + ______ HCl ---> ______ H2 + ______ ZnCl2 3. ______ Al + ______ SiO2 ---> ______ Si + ______ Al2O3 4. ______ Na + ______ HOH ---> ______ H2 + ______ NaOH 5. ______ Ni + ______ HgCl2 ---> ______ Hg + ______ NiCl2 6. ______ O2 + ______ CaC2 ---> ______ Ca + ______ CO2 7. ______ Al + ______ Fe2O3 ---> ______ Fe + ______ Al2O3 8. ______ F2 + ______ BN ---> ______ N2 + ______ BF3 9. ______ Al + ______ CuCl2 ---> ______ Cu + ______ AlCl3 10. ______ F2 + ______ AlI3 ---> ______ I2 + ______ AlF3 Write in the formulae for the products in the following single replacement reactions and then balance the equations. 11. ____ Al + ____ H2SO4 ---> ____ _____ + _____ ________ 12. ____ Cu + ____ AgNO3 ---> ____ _____ + _____ ________ 13. ____ Cl2 + ____ NaBr ---> ____ _____ + _____ ________ 14. ____ H2 + ____ Fe3O4 ---> ____ _____ + _____ ________ 15. ____ Cl2 + ___ CrBr3 ---> ____ _____ + _____ __________