here
advertisement

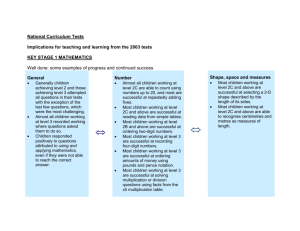
exam 1 scientific method chemical and physical properties and changes identify pure substance vs mixture element vs compound homogeneous vs heterogeneous names and symbols of elements 1 - 20, Al, Fe, Ni, Cu, Zn, Ag, Sn, W, Au, Hg, Pb periodic table identify elements by group number and by group name identify metals, non-metals, metalloids, transition metals metric system know and be able to apply nano, micro, milli, centi, deci, kilo prefixes including their abbreviations know the basic unit of each type o C = (5/9)(oF-32) will be given. Be able to use it to convert from oF to oC AND from oC to oF convert between Celsius and Kelvins significant figures count rounding in calculations scientific notation understand and interpret convert numbers to and from scientific notation unit algebra be good at it density q = mcΔT atoms charges and relative sizes of p+, no, e¯ understand and do calculations with atomic number, mass number understand isotopes, interpret isotope notation ( AZ X ) do calculations with the equation for average atomic mass quantum mechanical model of the atom fill in, interpret energy level diagrams electron configurations for atoms, ions noble gas shorthand valence electrons Lewis dot structures ions ions formation (predict charges) nomenclature of monatomic ions The following will be provided: periodic table 5 o C = ( o F - 32) ave. mass = (%1 )(mass1 ) + (% 2 )(mass2 ) + . . . 9 q = mcΔT 2.54 cm = 1 in. 1.057 qt = 1 liter 453.6 grams = 1 pound K = oC + 273.15 and any other information I think I should give you.