Reaction Predictions
advertisement

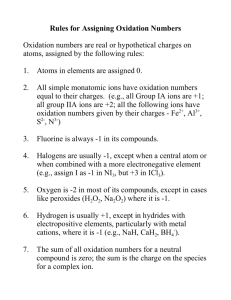
Reaction Predictions Most Commonly Used Cations and Anions Hydrogen H+ Sodium Na+ Potassium K+ Calcium Ca+² Magnesium Mg+² Iron (Ferrous) Fe+² Iron (Ferric) Fe+³ •Hydroxide OHˉ •Chloride Clˉ •Sulfide Sˉ² •Bicarbonate HCOзˉ •Carbonate COзˉ² •Sulfate SO4ˉ² •Phosphate PO4ˉ³ Cations/ Anions, contd. You can figure out the charge of an ion by using the periodic table. For Example: Alkali metals such as Lithium can easily lose an electron to become stable (just like a Noble gas) so taking away an electron give Lithium a +1 charge. On the other hand Halogens can easily accept an electron to become stable. Accepting an electron gives halogens a -1 charge. Practice What is the oxidation state of Oxide? What is the oxidation state of Iodide? What is the oxidation state of a Calcium ion? What is the oxidation state of a Lithium ion? Answers -2 -1 +2 +1 Net Ionic Equation To create a net ionic equation, you break apart all ionic molecules in a balanced molecular equation into their ions if they are soluble. If there are spectator ions, ions that appear on both sides of the equation, they cancel each other. Net Ionic Example Silver nitrate is mixed with potassium chromate 2AgNO3 + K2CrO4 → Ag2CrO4 + 2KNO3 Molecular Equation 2Ag+ + 2NO3ˉ + 2K+ + CrO4-2 → Ag2CrO4 + 2K+ + 2NO3-2 Complete ionic equation 2Ag+ + CrO4-2 → Ag2CrO4 Net Ionic Equation Solubility Rules NO3 all nitrates are soluble - - CH3COO or C2H3O2 all acetates are soluble except AgCH3COO ClO3 all chlorates are soluble Cl all chlorides are soluble except AgCl, Hg2Cl2, PbCl2 - Br all bromides are soluble except AgBr, PbBr2, Hg2Br2, and HgBr2 I all iodides are soluble except AgI, Hg2I2, HgI, and PbI2 - Solubility Rules, contd. SO4¯² all sulfates are soluble except BaSO4, PbSO4, Hg2SO4, CaSO4, AgSO4 and SrSO4 Alkali metal, cations, and NH4 – all are soluble H+ all common inorganic acids and low molecular mass organic acids are soluble (In)Soubility Rules, contd. - CO3 ² all carbonates are insoluble except those of alkali metals and NH4 CrO4 ² all chromates are insoluble except those of alkali metals, NH4, CaCrO4, and SrCO4 OH all hydroxides are insoluble except those of the alkali metals, NH4, Ba(OH)2, Sr(OH)2, and Ca(OH)2 PO4 ³ all phosphates are insoluble except those of alkali metals and NH4 SO3 ² all sulfites are insoluble except those of alkali metals and NH4 S ² all sulfides are insoluble except those of alkali metals and NH4 Synthesis Synthesis occurs when two or more reactants combine to form a single product. There are several common types of synthesis reaction. You know it happens when you have: -A metal combines with a nonmetal to form a bianary salt. -A piece of lithium metal is dropped into a container of nitrogen gas. 6Li+ N2 2Li3N -Metal oxide and water forms a base (metallic hydroxide) -Solid sodium oxide is added to water. Na2O + H2O 2NaOH Synthesis, contd. Nonmetal oxide and water forms acids. Nonmetal retains its oxidation number. -Carbon dioxide is burned in water. CO2 + H2O H2CO3 Metallic oxides and nonmetallic oxides form salts. -Solid sodium oxide is added to carbon dioxide. Na2O + CO2 Na2CO2 Decomposition Occurs when a single reactant is broken down into two or more products. The reactions react to form basic compounds or elements. When a compound is heated or electrolyzed, it means that it is broken up into its ions. AB A+B Examples of Decomposition A sample of magnesium carbonate is heated. MgCO3 MgO + CO2 Molten sodium chloride is electrolyzed. 2NaCl 2Na + Cl2 A sample of ammonium carbonate is heated. (NH4)2CO3 2NH3 + H2O + CO2 Single Replacement Reactions that involve an element replacing one part of a compound. The products include the displace element and a new compound. An element can only replace another element that is less active than itself. (Look a activity series/ AP packet) A +BX B+AX Single Replacement Rules 1. 2. 3. Active metals replace less active metals from the less active metals’ compounds in aqueous solutions ex. 3Mg+ 2FeCl3—> 2Fe + 3MgCl2 Active metals replace hydrogen in water ex. 2Na + 2H2O—> H2 + 2NaOH Active metals replace hydrogen in acids ex. 2Li + 2HCl —> H2 + 2LiCl Single Replacement Rules, contd. 4. Active nonmetals replace less active nonmetals from their compounds in aqueous solutions ex. Cl2 + 2KI —> I2 + 2KCl 5. If a less reactive element is combined with a more reactive element in compound form, there will be no reaction ex. Cl2 + KF —> no reaction* * On the AP test reactions will ALWAYS have products; it will never be “no reaction.” Activity Series (Single Replacement) Metals Li, Ca, Na, Mg, Al, Zn, Fe, Pb, [H2], Cu, Ag, Pt Nonmetals F2, Cl2, Br2, I2, More active Less Active Double Replacement Two compounds react to form two new compounds. No changes in oxidation numbers occur. Each cation pairs up with the anion in the other compound. The “driving force” in these reactions is the removal of at least one pair of ions from solution. This removal of ions happens with the formation of a precipitate, gas, or molecular species. When a double replacement reaction doesn’t go to completion, it is a reversible reaction (no ions have been removed). AX+ BY AY+ BX How do you know a double replacement reaction occurs? The reactants will contain a(n): -gas -insoluble precipitate -molecular species *Remember– on the AP test the reaction will always occur Common Gases Released (Dbl. Repl.) H2S Any sulfide plus any acid forms H2S and a salt. CO2 Any carbonate plus any acid form CO3, water, and a salt. SO2 Any sulfite plus any acid form SO2, water, and a salt. NH3 Any ammonium plus a soluble hydroxide form NH3, water, and a salt. Acid/ Base Reactions (Dbl. Repl.) An acid and a base will react and form water and a salt. Hydrochloric acid is added to sodium hydroxide. HCl + NaOH NaCl + H2O Hydrolysis (Dbl. Repl.) It is the reverse of neutralization and results when a salt plus a water molecule yields an acid plus a base. Salt + water acid + base Key things to know about hydrolysis reactions: Salts of a strong acid plus a weak base will hydrolyze into an acidic solution. NH4+ +Cl- +H2O → H+ +Cl- + (NH)4OH Salts of a weak acid and a strong base will always hydrolyze to give a basic solution. +F- +H2O → K+ +OH- +HF K+ Salts of a strong acid and a strong base will never undergo hydrolysis and therefore make a neutral solution. Na+ +Cl- +H2O → Na+ +OH- +H+ Cl- Salts of a weak acid plus salts of a weak base may hydrolyze as an acid, base, or a neutral solution; the final result depends on the Ka’s and Kb’s of the acids and bases formed during the hydrolysis process. Disclaimer!! The spectator ions were not removed Examples of Dbl. Replacement Solutions of potassium bromide and silver nitrate are mixed. KBr + AgNO3 AgBr + KNO3 A solution of sodium sulfate is added to a solution of hydrochloric acid. Na2SO3 + 2HCl 2NaCl + H2SO3 Hydrolysis Sample Problems Try these: An aqueous solution of manganese (II) sulfate undergoes hydrolysis. Ammonium fluoride and water are mixed together. Hydrolysis answers MnSO4 + 2H2O → H2SO4 + Mn(OH)2 NH4F + H2O → HF + NH4OH Combustion (Organic Reacs.) An organic compound reacts with O2 to form water and carbon dioxide. If something is burned there is a combustion reaction. Methanol is burned in oxygen gas. 2CH3OH + 3O2 4H2O + 2CO2 Addition (Organic Reacs.) A halogen or hydrogen is added to an alkene or alkyne, breaking apart the double or triple bonds and forming single bonds. Fluorine is added to ethene F2 + CH2=CH2 CH2F-CH2F Substitution (Organic Reacs.) An atom attached to a carbon is removed and something else takes its place. Bromine is added to methane Br2 + CH4 CH3Br + HBr Oxidizing Agents (Redox Reacs.) Common Oxidizing Agents MnO4¯ in acidic solution MnO2 in acidic solution MnO4¯ in neutral or basic solution Cr2O7ˉ² in acidic solution HNO3, concentrated HNO3, dilute H2SO4, hot, concentrated Metallic ions (higher oxidation #) Free halogens Na2O2 HClO4 C2O4ˉ² H 2 O2 Products Formed Mn+² Mn+² MnO2(s) Cr+³ NO2 NO SO2 Metallous ions (lower oxidation #) Halide ions NaOH Clˉ CO2 O2 Reduction Agents (Redo Reacs.) Common Reducing Agents Halide ions Free metals Sulfite ions or SO2 Nitrite ions Free halogens, dilute basic solution Free halogens, concentrated basic solution Metallous ions (lower oxidation #) Products Formed Free halogen Metal ions Sulfate ions Nitrate ions Hypohalite ions Halite ions Metallic ions (higher oxidation #) Electrolysis (Redox Reacs.) An electrolysis reaction is a reaction in which a nonspontaneous redox reaction is brought about by the passage of current under sufficient external electrical potential. The devices in which electrolysis reactions occur are called electrolytic cells. In theory, E° values (Standard Reduction Potentials) can be used to predict which element will plate out at a particular electrode when various solutions are combined. (B&L text) Rules for Predicting Cathode Reactions (Reduction) When a direct electric current is passed through a water solution of an electrolyte, two possible reduction processes may occur at the cathode. The cation may be reduced to the corresponding metal. Mn+ + ne- M(s) (reaction 1) n = (charge of cation) Water molecule may be reduced to elementary hydrogen 2H2O + 2eˉ H2 + 2OHˉ (reaction 2) Rules for Predicting Cathode Reactions, contd. For salts containing transition metal cations, which are relatively easy to reduced compared to water, reaction #1 will occur at the cathode (and the transition metal will plate out). Mn+ + ne- M(s) If the cation is representative metal, the water molecules will be easier to reduce compared to the cation, and reaction #2 will occur at the cathode, producing hydrogen gas and hydrogen ions. 2H2O + 2eˉ H2 + 2OHˉ Rules for Predicting Anode Reaction (oxidation) The oxidation process that occurs at the anode of an electrolytic cell operating in aqueous solution may be one of two oxidation processes. The anion may be oxidized to the corresponding nonmetal. - 2Xˉ X2 + 2eˉ (reaction 1) Water molecules may be oxidized to elementary oxygen. - HOH ½ O2 + 2H+ + 2eˉ (reaction 2) Rules for Predicting Anode Reactions, contd. For salts containing iodide, bromide, or chloride ions, it is usually easier to oxidize these nonmetals rather than water. It will be found that the nonmetal is formed at the anode. When the anion present is any other ion that is more difficult to oxidize than water, Reaction #2 will occur at the anode producing elementary oxygen and aqueous hydrogen ions. Example Electrolysis Reactions Copper (II) chloride in water Cu+2 + 2Clˉ Cu + Cl2 2. Copper (II) sulfate in water Cu+2 + HOH Cu + ½ O2 + 2H+ 3. Sodium chloride in water 2Clˉ + 2HOH H2 + Cl2 + 2OHˉ 4. Sodium sulfate in water 2HOH 2H2 + O2 1. Metals w/ Multiple Oxidation Levels (Redox Reacs.) These metals can change their oxidation state in a redox reaction Antimony (III) or (V) Bismuth (III) or (IV) Cerium (III) or (IV) Chromium (II) or (III) Cobalt (II) or (III) Copper (I) or (II) Gallium (I) or (II) or (III) Germanium (II) or (IV) Gold (I) or (III) Iron (II) or (III) Lead (II) or (IV) Mercury (I) or (II) Nickel (II) or (III) Thallium (I) or (III) Thorium (II) or (IV) Tin (II) or (IV) Tin (II) sulfate is added to iron (III) sulfate SnSO4 + Fe2(SO4)3 Sn(SO4)2 + 2FeSO4 Complex Ion Reactions Nomenclature is on pages 23-27 of The Ultimate Chemical Equations Handbook There are a lot of very complicated types of these reactions, but, for all intensive purposes and for the AP test, you only need to be familiar with those reactions pertaining to ammonia and water. In a complex ion reaction, ligands will attach to a transition metal ion. There will usually be twice as many ligands as the metals oxidation number Complex Ion Reactions, contd. These reactions usually occur in a concentrated solution of the ligand. Copper chloride (II) is added to a concentrated solution of ammonia Cu2+ +NH3 [Cu(NH3)4]2+ Common Reaction Terms Electrolysis: Electricity is run through a compound, resulting in a change of oxidation states. Hydrolysis: The reaction of a salt with water to form molecular species. Salts of a strong acid + a weak base will always hydrolyze to give an acidic solution. Neutralization: Acid and base react to form a salt and water. Catalyst: A molecule that speeds that speeds a reaction but that does not appear in the reaction. Oxidation number: the charge that it would have if all the ligands (atoms that donate electrons) were removed along with the electron pairs that were shared with the central atom Common Reaction Terms, contd. Precipitate: an insoluble substance formed by the reaction of two aqueous substances. Anode: the electrode where oxidation occurs an ox Cathode: the electrode where reduction occurs red cat By: Will Lambert, Adam Robinson, Michelle Klassen, and Tori Waldron (APChem ‘06-’07)