19-1 - chemrocksium
advertisement

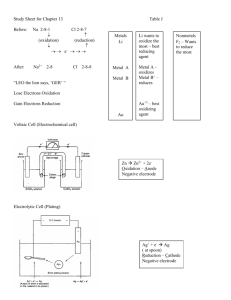
19-1 The oxidation number of an atom in a substance is equal to the charge that the atom would have if the electrons in each bond belonged to the more electronegative atom. *Are not true ionic charges. *Are used to keep track of the electrons Rules for Assigning Oxidation Numbers 1. Uncombined element is zero 2. Monatomic ion equals its ionic charge. 3. More electronegative element gets negative oxidation number 4. Oxidation numbers are per atom. 5. The algebraic sum of the individual oxidation numbers of all the atoms in the formula for a neutral compound is zero. 6. For a polyatomic ion, they equal the charge of an ion. 7. hydrogen is =1 unless with a metal then –1. +/- Goes in front for oxidation numbers. In back for true ionic charges. OXYGEN OXIDATION REDUCTION GAIN LOSS OXIDATION NUMBER INCREASE DECREASE ELECTRONS LOSS GAIN *Oxidation and reduction must occur together (because of law of conservation of mass and/or matter) Oxidation-Reduction Reaction- (Redox Reaction) - a reaction where electrons transferred between the reactants. Half reaction- the part of the reaction involving oxidation or reduction alone. 19-2 Half reaction method- In acidic solution. steps 1. separate into half reactions 2. balance all elements but H and O 3. balance O using water 4. balance H using H+ 5. balance charge using e6. make sure e- cancel using lowest common multiple 7. add 2 half reactions and cancel like terms Changing it to basic solution---- 1. balance in acid solution first 2. add the same number of hydroxide as you have H+ to both sides 3. cancel some water to get final answer Remember when you cancel like terms you can only cancel what you added. Specifically H+ and e- and water. *The fundamental principle in balancing redox equations, is that the number of electrons lost in an oxidation process (increase in oxidation number) must equal the number of electrons gained in the reduction process. (decrease in oxidation number) 19-3 Oxidizing agent- causes the oxidation of another substance by accepting electrons from that substance. Therefore, the oxidizing agent contains the element that is reduced. Reducing agent- causes the reduction of another substance by donating electrons to that substance. Therefore, the reducing agent contains the element that is oxidized. Types of Redox Reactions 1. Direct combination reaction 2. Decomposition reaction 3. Single replacement reaction *The above are all redox because they have an uncombined element on one side (ox#= 0) and then the same element in compound on other side of the reaction (ox# doesn’t = 0) Activity series of metals is a listing that ranks metals according to their relative reactivity. (page ) *Used for single replacement reactions *An element will only replace an element less reactive than itself, literally means = one below it on the chart. *More reactive at top 20-1 ElectrochemistryBranch of chemistry that deals with electricity related applications of oxidation–reduction reactions. Two types of electrochemical cells 1. Voltaic cell- those that produce electricity as the result of spontaneous redox reactions (Daniell cell) 2. Electrolytic cell- eletrochemical cells in which electrical energy drives nonspontaneous redox reactions. Parts of Voltaic Cell Salt bridge- tube that contains an electrolyte solution and connects the two solutions of a voltaic cell; allows ions to move from one compartment to another without mixing the solutions. (porous barrier) Electrode- substance through which electrons enter or exit electrochemical cells. Made of metal and provides surface on which oxidation and reduction occurs. *rest will be on diagram. Two Types of Electrodes 1. Anode- electrode where oxidation occurs 2. Cathode- electrode where reduction occurs Keep vowels together anode + oxidation and keep your constantans together. Cell Notation- shorthand way to write parts of voltaic cell. *generic form template anode| anode solution || cathode solution| cathode 20-2 Voltaic cell- those that produce electricity as the result of spontaneous redox reactions (Daniell cell or galvanic cell) Electrons flow from anode to cathode. * Batteries that can be recharged have equations that are easily reversed *Common dry cell- everyday type battery *Alkaline dry cell- more expensive, better ingredients *Lead storage- car battery * Nickel -cadmium---> rechargeable Fuel cell- use in space shuttle, continuous fuel supply, too expensive Corrosion occurs when iron is exposed to oxygen and water. Prevent corrosion by using a sacrificial anode by galvanizing item. Cell potential-the ability of a cell reaction to move electrons through a wire from one electrode of a voltaic cell to another is described by this quantity. (Measured in volts) Voltage- a measure of the cells ability to drive an electric current through a wire and there by do work. Standard electrode potential- represents the tendency of that half reaction to occur and are based on a comparison with the standard hydrogen electrode potential of 0.00 V. Symbol is Eo *Can be either oxidation or reduction. Chart is on page 664. Eocell = Eoreduction + Eooxidation *hydrogen is the standard by which the rest were measured so his value is zero. *Everything on the chart is reduction. If you need oxidation you reverse the sign on the voltage and flip the reaction. *when you multiple the reaction by a factor to cancel electrons E ocell remains the same. 20-3 Electrolytic cell- electrochemical cells in which electrical energy drives nonspontaneous redox reactions. * Example- electroplating, object plated is cathode. 3 main differences between voltaic cells and electrolytic cells 1. Electrolytic cells need a battery for a power source. Voltaic cells make the energy. 2. Reaction in electrolytic cells is nonspontaneous; in a voltaic cell it is spontaneous. 3. Electrolytic cells= anode is positive, cathode is negative. Voltaic cells= anode is negative, cathode is positive. Electrolysis- process by which electricity is used to bring about a nonspontaneous chemical reaction. *Used to purify metals