I. The Atomic Concept:
advertisement

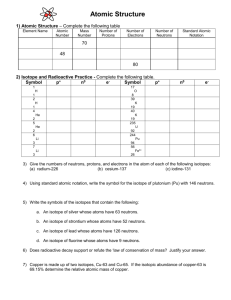
CHEM 163 EXPERIMENT NUCLEAR CHEMISTRY Name_________________ Isotopes and Radioactivity After completing this learning activity packet, you should be able to do the following: (1) Define what isotopes are and give examples. (2) Write standard isotope symbol for an atom when the numbers of proton, neutron and electron are given; tell the numbers of proton, neutron and electron present in an atom, when given its standard isotope symbol. (3) Name and describe three types of natural radioactivity in terms of standard isotope symbol, mass, charge and penetration power. --------------------------------------------------------------------------------------------------------------------------------- I. The Atom and Its Nucleus This description of an atom is based on the nuclear atomic model, first proposed in 1911 by Ernest Rutherford, a New Zealander doing research in England. Rutherford developed the nuclear atomic model from the results of his famous gold-foil experiment. He bombarded a piece of gold foil with small, positively charged alpha particles and traced these particles. As shown in Figure 1, he found that, as expected, most of the particles shot straight through the foil. However, to his Figure 1. Rutherford's Gold-Foil Experiment a bounceback particle a deflected particle stream of fast moving alpha particles Gold foil, a few layers of atoms thick. Close-up view of one gold atom; size of the nucleus greatly exaggerated. amazement, a small percentage of the particles deflected to the sides, and a few of them even bounced back! He concluded that the deflected alpha particles must have encountered positively charged centers in the gold foil, which deflect them from a straight course due to like-charge repulsion. A few alpha particles may have collided head-on with these centers, which must be dense or massive enough to bounce the alpha particles backward. Based on the small ratio of particles affected by the nuclei, he estimated the ratio between the radii of the nucleus and the atom as being 1: 100,000. Further experimental evidences to-date confirm Rutherford's idea. In addition, it has been determined that a proton and a neutron weigh approximately the same, and each of them weighs 1,837 times more than an electron. The mass of a proton is defined as 1 atomic mass unit, or 1 a.m.u. The mass of an electron is therefore: 1 1,837 = 0.000544 a.m.u. In addition, a proton carries +1 charge, an electron carries -1 charge, and a neutron has no charge. Answer the following questions concerning the atom and its nucleus. Q1. A gold atom contains 79 protons, 118 neutrons and 79 electrons. (a) What is the overall electrical charge of a gold atom? _________ Explain or show calculation. _______________________________________________________________________________________ (b) What is the electrical charge of the nucleus of a gold atom? _______ Explain or show calculation. _______________________________________________________________________________________ (c) What is the mass of a gold atom in terms of a.m.u.? ________ Explain or show calculation. _______________________________________________________________________________________ (d) What is the mass of the nucleus of a gold atom in terms of a.m.u.? ______ Explain or show calculation. _______________________________________________________________________________________ Q2. An alpha particle consists of 2 protons, 2 neutrons and no electron. (a) What is the overall electrical charge of an alpha particle? ________ Explain or show calculation. _______________________________________________________________________________________ (b) What is the mass of an alpha particle in terms of a.m.u.? ________ Explain or show calculation. _______________________________________________________________________________________ (c) Compare the mass of the gold nucleus with that of the alpha particle. Explain why it is reasonable to expect that an alpha particle will bounce back from a gold nucleus in a head-on collision of the two, as observed in the gold-foil experiment. _______________________________________________________________________________________ II. Different Types of Atoms A. Isotopes The number of protons is unique in all the atoms of the same element. It is defined as the atomic number of the element, and is given for each element in the periodic table. The atoms of the same element, however, may have different numbers of neutrons. Isotopes are atoms of the same element with different numbers of neutrons. Q3. (a) How are the isotopes of an element similar to each other?__________________________________ (b) How do the isotopes of an element differ?__________________________________________________ B. Mass Numbers and Averaged Atomic Mass The sum of the protons and the neutrons in an atom is defined as the mass number. This number is unique for each isotope of an element. An isotope is often identified by the symbol of an element followed by a specific mass number. For example, Ne-20 denotes an isotope with mass number = 20 or Ne-22 denotes another isotope with mass number = 22. Mass number approximately equals the mass of the atom, because each proton and neutron weighs approximately 1 a.m.u., and the mass of electrons is negligible. Q4. Calculate the mass numbers of the three isotopes of hydrogen from lecture: _____, ______ and _______ Q5. What is the mass number of the most abundant isotope of carbon? ____________ Q6. Co-60 identifies an isotope of the element named ______________ , 60 denotes ____________. C. Standard Isotope Symbols The notation as Si-30 or Silicon-30 identifies an isotope, but more information concerning the atom is provided by the standard isotope symbol. The standard isotope symbol specifies in a concise way all the following information of an atom: its elemental symbol, mass number, atomic number and the electrical charge. Each information is positioned in a particular location in the isotope symbol, as shown in Figure 1, next page. mass number (upper left corner) A atomic number (lower left corner) Z element symbol (main body) net charge (upper right corner, not indicated if the net charge is zero) # neutrons (lower right corner) Figure 1. Standard Isotope Symbol The standard isotope symbols are: 11 H 01 and 42 He 2 2 . Answer the following questions Q7. (a) In the symbol 11 H 01 , the atomic number is ________ , (b) the mass number is ________ and (c) the net charge of this isotope is ______ . The symbol 42 He 2 2 , indicates the isotope's atomic number is (d) ______, its mass number is (e) _____, and the net charge is (f) _______ . Q8. Calculate the numbers of subatomic particles present from isotope symbols. Remember that: Number of protons = Atomic Number Number of neutrons = Mass number atomic number Number of electrons = Atomic number - net charge (a) The symbol, 11 H 01 denotes a hydrogen isotope with: ______ proton, ______ neutron, and _______ electron. (b) The symbol 42 He 2 2 denotes: element name ____________ with _____ p+, ______ n0 and ________ e-. Q9. Complete Table 1 with appropriate isotope symbols and numbers of subatomic particles. Table 1. Standard Isotope Symbols Isotope Symbol number of number of number of protons neutrons electrons 2 2 2 30 26 mass number atomic number 26 197 Au 79 118 60 Co 27 33 40 Ca 2 20 20 30 Isotope Symbol number of proton number of neutron 28 65 numbr of electron mass number atomic number 16 O 2 8 8 78 54 53 35 Cl1 17 18 III. LAB EXCERISE Nuclear Changes and Radioactivity A nuclear change involves changes in the nucleus of the atom. A nuclear change will involve often with the lost or gain of protons and /or neutrons. An atom of one element may turn into that of another element with a different atomic number, and/or mass number. There are three types of natural radiation, named after the first three letters of the Greek alphabet: alpha (), beta () and gamma (). These can be detected by a Geiger radiation counter. In general terms, a Geiger tube collects the energetic particles or rays of radiation, and these generate electrical impulses in the tube. The electrical impulses are noted by a counter and reported with a digital display, a meter, or as audible clicks. Procedures: 1. Connect a radiation monitor to DIG channel port on the LabQuest and choose NEW from the File menue. a. See instructor if Monitor does not appear in LabQuest screen 2. Set up data-collection mode a. On screen, tap Mode. Change mode to Events with Entry b. Enter the Name (Reading) and leave the Units field blank. A value of 50 seconds/interval is acceptable c. Select OK 3. Set up the monitor a. Place the source near the metal screen. To test an absorber, place absorber between the source and the screen b. Use approximately the same position for the sources each time, with and without an absorber. The plastic disc sources emit the most radiation from the underside. 4. Determine background count rate a. Move all sources away from the monitor b. Start data collection to prepare the system to collect data c. Tap Keep. There will ba a 50 sec delay for counting d. Enter 0 as the Reading value to indicate for background count. Select OK to store data pair. 5. Test beta source a. Place beta cource near monitor, as directed in Step 3 b. Tap Keep. Counts are taken for 50 sec c. Enter 1 as the Reading value. Select OK d. Place a single sheet of paper between the beta source and the monitor e. Select Keep. When the count is complete, enter 2 as Reading value, select OK f. Repeat process using aluminum as the shield. Select Keep, enter 3 as Reading value, select OK g. Stop data collection 6. Record the data in the data table 7. Start data collection and repeat process with the Gamma source DATA TABLE Background (cpm) 1st count No Shielding Background Beta source Gamma source Alpha source 2nd count Paper Sheild 3rd count Al Shield Shield average cpm Shield Shield Q10. Complete the following table concerning the three types of natural radiation you studied. Table 3. Compositions of Three Types of Natural Radiation standard isotope symbol 4 2 2 4 2 He 2 or 2 2 0 1 1 e or 0 1 1 0 0 0 0 name of radiation number of proton(s) present number of neutron(s) present number of electron(s) present Q11. Answer the following questions. You may not have be able to collect data for the Alpha sourc (a) Which radiation, alpha, beta or gamma, has highest penetration power (not cut off by the shields)? ____ (b) Which radiation, alpha, beta or gamma, has the highest mass?__________________________ (c) Which radiation, alpha, beta or gamma, carries a negative electrical charge?___________________ (d) Which radiation, alpha, beta or gamma, is cut off completely by the lead shield? __________________ FURTHER STUDIES Isotope Symbols Q12. Write standard isotope symbols for the following ions or isotopes: (a) barium with 54 electrons & 81 neutrons (b) oxygen with -2 charge and 10 neutrons (c) lithium with 4 neutrons (d) An ion with 2p+1 and 2n0, zero e-1 (e) An ion with 35p+1, 45n0 and 36e-1 (f) An ion with 56 p+1, 81n0 and 54e-1 Q13. (a) Complete the following table. standard isotope symbol # proton # neutron # electron 12 13 12 26 26 mass number atomic number magnesium-25 58 iron-58 65 Cu 2 29 36 standard isotope symbol name copper-65 # proton # neutron # electron mass number atomic number 92 235 92 name 14 C 6 8 37 Cl 17 20 17 18 18 (b) Which of the above are isotopes to each other?_____________________ and __________________. Averaged Atomic Weight Isotopes are atoms of the same element with different numbers of neutrons. For example, chlorine has two major isotopes: 75% is chlorine-35 with 18 neutrons, and 25% is chlorine-37 with 20 neutrons. (These percentages are called % natural abundance.) Chlorine-35 has the atomic mass of 35 a.m.u. and chlorine-37 has the atomic mass of 37 a.m.u. The mass reported for chlorine in the periodic table is neither 35 nor 37; it is a weighted average of these two numbers. The weighted average is calculated by taking 75% of mass 35 plus 25% of mass 37: (35 amu x 75%) + (37 amu x 25%) = (35 x 0. 75) + (37 x 0. 25) = 35. 5 amu Q14. Answer the following questions. (a) mass of chlorine on the Periodic Table: ______. (b) Is it close to 35. 5? _____ (c) Try a similar calculation for another element, copper. Given the following information: Copper has two major isotopes: 69.1% of copper-63 and 30.9% of copper-65. ( ________ amu ________%) + ( ________ amu _______%) = (_______ ________) + (________ _________) = _______________ amu. How does this value compare to the reported mass in the table?___________ Natural Abundance of Different Isotopes Q15. Radioactivity is of two types, Natural and Induced radioactivity. Define each type. a. Natural radioactivity: ______________________________________________________________ b. Induced radioactivity: ______________________________________________________________ c. A stable nucleus may be coverted to an unstable nucleus by _______________________ with highenergy particles or radiation. d. New elements have been made by bombarding nuclei of heavy elements with nuclei of light elements. Elements with atomic number greater than 92, are made this way. What is the term for these elements? ______________________________________________ Isotopes are atoms of the same element with different neutron numbers. Almost every element has two or more isotopes in existence naturally. An element exists in nature with a certain percentage of its atoms being of one isotope, and other percentages being of other isotopes. This percentage break-down of isotopes for each element has been compiled and tabulated in the CRC Handbook of Chemistry and Physics. Obtain a copy of the CRC Handbook of Chemistry and Physics. In the index, look up the entry: “Isotopes abundance, % natural abundance table”, and locate the complete table listing this information. You will see a detailed table of isotopes listed in order of atomic number. Acquaint yourself with the natural isotopes of the following common elements with the information given in this table. Q16. Complete the following. (a) The CRC Handbook I used is of the _______ edition, published in the year _____________ . (b) How many different isotopes of oxygen are listed in this Handbook? _________ (c) Which isotope of oxygen has the highest percentage of natural abundance? ____________ Note: natural abundance refers to the natural occurrence of an isotope in nature. The isotope with highest natural abundance is the most common or predominant isotope of that element. Which is the most common natural isotope of nitrogen? _______ What is its % natural abundance? ___ (d) The isotope of hydrogen H-1, is the most common in nature. Its % natural abundance is ____________ . (e) The isotope H-2 (named deuterium), is rare in nature. Its % natural abundance is ____________ . Questions For Review what you have learned: Isotopes and Radioactivity KEY TERMS: you have read or used the following important terms or concepts. Write a sentence or two to describe each of them in your own words first. 1. (a) What are the three types of natural radiation? ___________________________________________ (b) Describe each one in terms of mass, charge and penetration power.________________________________ ____________________________________________________________________________________________________ 2. What is background radiation? ________________________________________________________________ ____________________________________________________________________________________________________ 3. Describe what is meant by Nuclear Change? _____________________________________________________ ___________________________________________________________________________________________ Explain, analyze, apply, evulate what you have learned. 4. What information can be obtained from a standard isotope symbol? __________________________________ ___________________________________________________________________________________________ 5. How does the gold foil experiment provide evidences for the nuclear model of the atom?__________________ ___________________________________________________________________________________________ 6. (a) Is it true that one can avoid alpha radiation by wearing gloves and regular clothing? ______ (b) Explain your answer to (a)? _______________________________________________________________ ____________________________________________________________________________________________________ 7. (a) Is it true that one must use a lead shield to avoid beta radiation?__________ (b) What is the reasoning for your answer? ______________________________________________________ Absorber Dector Radiation Source Cable Plug Counter screen Distance marked that dector is from the source absorders