Reactions_of_Ethylene_Answers
advertisement
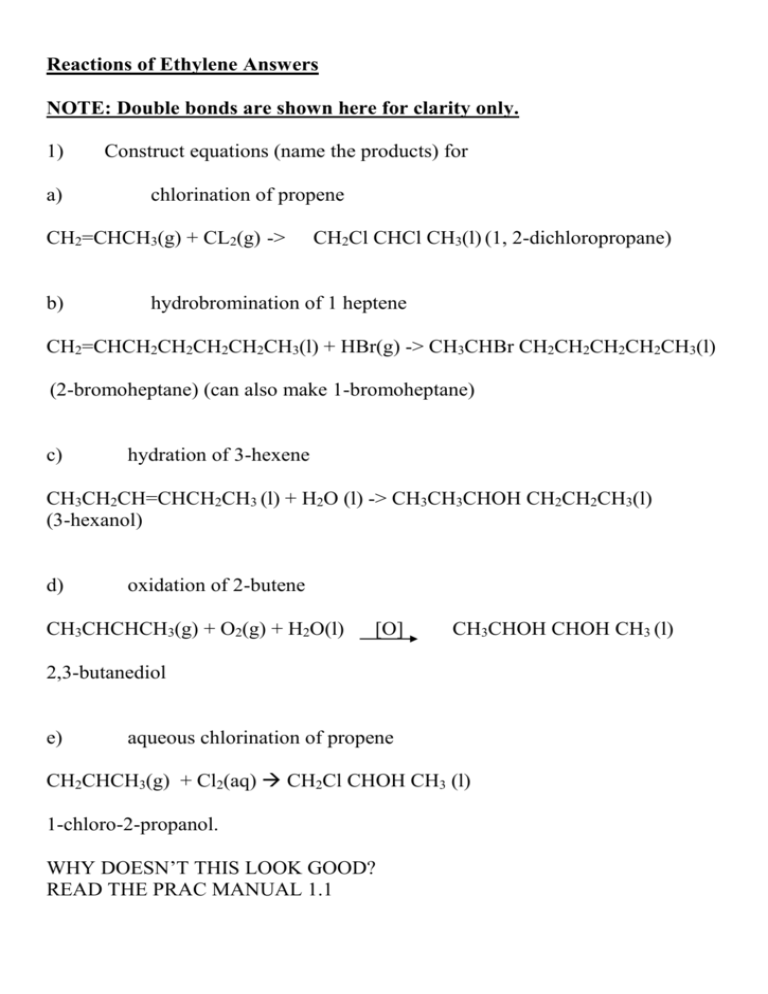
Reactions of Ethylene Answers NOTE: Double bonds are shown here for clarity only. 1) a) Construct equations (name the products) for chlorination of propene CH2=CHCH3(g) + CL2(g) -> b) CH2Cl CHCl CH3(l) (1, 2-dichloropropane) hydrobromination of 1 heptene CH2=CHCH2CH2CH2CH2CH3(l) + HBr(g) -> CH3CHBr CH2CH2CH2CH2CH3(l) (2-bromoheptane) (can also make 1-bromoheptane) c) hydration of 3-hexene CH3CH2CH=CHCH2CH3 (l) + H2O (l) -> CH3CH3CHOH CH2CH2CH3(l) (3-hexanol) d) oxidation of 2-butene CH3CHCHCH3(g) + O2(g) + H2O(l) [O] CH3CHOH CHOH CH3 (l) 2,3-butanediol e) aqueous chlorination of propene CH2CHCH3(g) + Cl2(aq) CH2Cl CHOH CH3 (l) 1-chloro-2-propanol. WHY DOESN’T THIS LOOK GOOD? READ THE PRAC MANUAL 1.1 2) Propose a method for the preparation of (include an equation) a) b) c) d) e) 2-chlorobutane (addition of HCl (g) to 1-butene or 2-butene) 2,3-dichlorobutane (addition of CL2(g) to 2 butene) 3-bromo-2-butanol (addition of Br2(aq) to 2 butene) 2-butanol (addition of water to 1-butene) 2,3-pentanediol (oxidation of 2-pentene) Using what you have done already, and the answers to Q1 you should be able to write the balanced equations now I have given you the reagents.