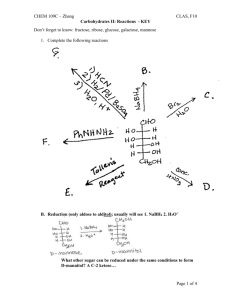
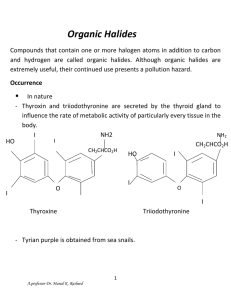
Acetal Formation Major concepts A hemiacetal is a functional group
advertisement

Acetal Formation Major concepts A hemiacetal is a functional group in which a carbon is bonded to a hydroxyl group and an -OR group The acetal is a functional group in which a carbon atom is bonded to two –OR groups Acetal formation is a condensation reaction between two hydroxyl groups and a ketone or aldehyde in which water is lost. Vocabulary Acetal Hemiacetal Diol Students should be able to: Identify the acetal and hemiacetal groups in a molecule Explain the mechanism of acetal formation Predict the products of acetal formation given starting materials Given an acetal, do a retrosynthesis to the corresponding carbonyl and alcohols or diol Be able to compare/contrast acetal formation (a condensation reaction ) with aldol condensation and imine formation. Daily Problems 1. Which of these molecules can serve as an electrophile in acetal formation? Explain. O O O HO O O O O Cl H H2N 2. Which of these molecules can serve as the nucleophile in acetal formation? 3. Propose a mechanism for the formation of this hemiacetal and then acetal. (You may use your notes to anwer this question.) What type of reaction is the formation of the hemiacetal? How could you describe the second half of the reaction? How could you describe the overall reaction? O CH3OH solvent H HO OCH3 H3CO OCH3 catalytic H+ H 4. Identify any hemiacetal and acetal functional groups in this molecule: H 5. Draw the imine products that form when acetaldehyde is condensed with each of these amines with catalytic acid. 6. Draw the imine products that form when methylamine is condensed with each of these aldehydes/ketones and catalytic acid. 7. Retrosynthesis: Give the structure of the alcohol(s) and the carbonyl compound necessary to make these acetals under acidic conditions. Cumulative Problems: 8. What is a condensation reaction? What are three types of condensation reactions that we have learned? Compare and contrast them. 9. Predict the products of these imine formations and aldol condensations. How are they similar to each other. (Hint: Use the example strategy of identifying the nucleophile and electrophile and drawing the condensed product.) 10. Draw the product of these condensation reactions and label the type of condensation reaction. Extension problem: 11. Find the hemiacetal. Do the retrosynthesis to get back to the alcohol and the aldehyde. (Hint: Don’t try to move the carbon chain—keep it in the same orientation.