lab starter - Virtual Homeschool Group
advertisement

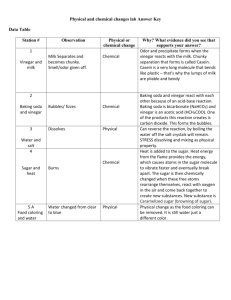
Moore, Timothy Moore, Timothy Instructor: Mrs. Tammy Moore Class: VHSG Online Chemistry 26 May 2009 EXPERIMENT 10.2 Acid/Base Titration Acid/base titration using red cabbage indicator Abstract [What you will put here: Summarize the whole report in one, concise paragraph of about 100-200 words. You cannot write the abstract until after you've completed the report. Because it assumes that you will speak to your conclusion or what you learned in brief.] Introduction [What you will put here: In this introduction, some background is provided for the reader (sources are noted in the reference section at the end of the report).Typically, the introduction states the problem to be solved or the experiment to be performed and explains its purpose and significance. It also provides whatever background theory, previous research, or formulas the reader needs to understand to perform the experiment (or solve the problem).] Methods and Observations Materials Red cabbage leaves 2 beakers (or one pot to boil water in and one short/fat glass) Source of heat to boil the water Graduated cylinder (measuring cup can be used but I less accurate) Powdered drain cleaner (Draino) or scouring powder (Comet) Pipette (medicine dropper) Clear ammonia (cleaning supply isle of a grocery store should have it) Moore, Timothy Clear vinegar (do not used colored vinegar) Mass scale Distilled water White sheet of paper (no lines) Stirring rod (or small spoons) MAKING THE RED CABBAGE pH INDICATOR: I rinsed all my equipment with distilled water. Tap water’s minerals can push tap water away from full neutrality of pH. I then brought about 50 mL of distilled water and chopped cabbage leaves to a boil for 5 minutes. I wanted the cabbage in pieces so that there would be a lot of surface area so as much of the red/purple color could be released into the water as possible while still having large enough pieces to make it easy to catch the pieces in a strainer so I could collect the liquid portion easily. The liquid portion is my pH indicator. PREPARING THE BASE: I added 10 mL (2 tsp) of clear ammonia, 90 mL (about ½ c) of water, and half of the indicator solution into a beaker. PREPARING THE ACID: I took the mass of 50 mL of vinegar and entered the figure into my data chart (see the data section of this report). [If you have a scale that will tare, use that to eliminate the mass of the graduated cylinder from your calculations. If you do not have a scale that will tare, you will need to measure the mass of your cylinder then subtract that from the total mass of the solution you will make so that your calculations will only have the mass of the solution. Explain what you did here based on your scale]. I calculated the vinegar’s density by dividing the mass by the volume. That value is also in the data table. CALIBRATING MY PIPETTE (MEDICINE DROPPER): Since a calibrated titration pipette is beyond the budget of my lab equipment, I substituted using an eye dropper pipette. This doesn’t have the convenient measurement markings and stop cock valve of the real thing, but will be sufficient for my purposes. To use my substitute, I must count drops to know Moore, Timothy how much is added and know about how much solution is in each drop. I was able to get an average volume per drop from my pipette. I didn’t have a measuring device precise enough to measure such a small volume of liquid, but I did have one that would measure 100 drops. I counted 100 drops, took the volume of those 100 drops, and divided that by 100 to get the volume of one drop. The data was added the data section [Note: if your graduated cylinder or whatever device you are using still cannot measure the volume even with 100 drops, then use a larger number and divide by that instead of 100]. PERFORMING THE ROUGH TITRATION: The rough titration got me close to the value that I needed and then I shifted to going slower to get a more exact measurement. Otherwise, it would have taken a very long time going drop by drop from the very beginning. So, for rough titration, I squirted full dropper’s amounts of the vinegar (acid) solution into the ammonia (base/indicator) solution. I saw the very first hint of a color change toward pink. If stirred, the color readily disappeared again. I looked at the graduated cylinder and computed how much I had added by subtracting what I originally had from what I had at that point. This was added to the data in the data section. REACHING THE END POINT: The rough titration got me close to the end point. I then began counting drops as I continued to add the vinegar. I stirred when I saw a hint of pink. If the color disappeared, I continued adding and counting drops. When I reached the point where stirring did not make the pink disappear, I knew I had reached the end point. I recorded the number of drops that I added in total to get it from the rough titration point to the end point and added that value to my data. DATA Vinegar’s Density: Volume of 1 drop of vinegar from my pipette (eyedropper): Total volume of vinegar added during the rough titration: Total number of drops times the volume in one drop: Total volume of vinegar added (sum of the two previous data lines): Moore, Timothy CALCULATIONS I computed the molarity (concentration) of the titrant, the ammonia with the following calculations. For every mole of acid I added, one mole of base got ‘eaten up’ because the equation for the reaction is C2H4O2 + NH3 NH41- + C2H3O21I can use the data I collected to determine how many moles of vinegar was used and then that would be the number of moles of ammonia I started with. Calculating the number of moles of vinegar: 1. To find the number of grams, I multiplied the density of vinegar by the number of mL added to the reach endpoint: [enter your calculations here] 2. Since the vinegar is only about 5.00% of the vinegar, I multiplied the value in step 1 by 0.05 [show your calculation here] 3. I then calculated from grams to moles. Since the ratio of acid to base is the same in the chemical equation for this reaction. That is how many moles of ammonia (base) we have too. [show your calculations here – gram to mole calculations was a big part of module 6 Review the VoiceThreads for that module if you need to] 4. Now we finally get to find the concentration. The concentration of a base is the number of moles of the base divided by the volume (in liters) of the solution. [Show your calculations. The volume of ammonia was 10 mL(see the Preparing the Base’ section to see where we got the 10mL from) Conclusion My observations [You must explain, analyze, and interpret your results, being especially careful to explain any errors or problems. This is probably the single most important part of the report, since it is here that you demonstrate that you understand and can interpret what you have done. Read Dr. Wile’s explanation of the experiment in the text, if needed. Just don’t copy it word for word. ] References [Use MLA citation standards. This is commonly used in lab reports, but APA is used in some colleges too. I encourage you to do some of your own research too and add to the introduction section of the lab report from what you learn.






![CSUS - CH6A, [Mass percentage of acetic acid in vinegar] Instructor](http://s3.studylib.net/store/data/007937173_1-e0c351dc5daed812e8e6ea99de8c0e8a-300x300.png)