MYP 10 Chemistry 2012-13 Atomic Sructure Worksheet Name
advertisement

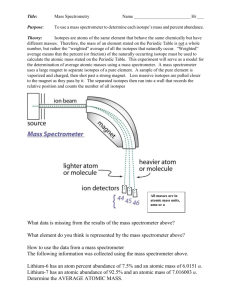
MYP 10 Chemistry 2012-13 Atomic Sructure Worksheet Name: _________________________________ ( ) Class: _________ Date: _____________ _________________________________________________________________________________ 1. The element bromine exists as the isotopes 79Br and 81Br, and has a relative atomic mass of 79.90. (a) Copy and complete the following table to show the numbers of sb-atmic particles in the species shown. An atom of 79Br An atom of 81Br Protons Neutrons Electrons (b) State and explain which of the two isotopes 79 Br and 81Br is more common in the element bromine. [SL paper 2, Nov 05] 109 2. The element silver has two isotopes, 107 47𝐴𝑔 and 47𝐴𝑔, and a relative atomic mass of 107.87. (a) Define the term isotope. + (b) State the number of protons, electrons an neutrons in 107 47𝐴𝑔 . (c) State the name and the mass number of the isotope relative to which all atomic masses are measured. 3(a) List the following types of electromagnetic radiation in order of increasing wavelength (shortest first) I. II. III. IV. Yellow light Red light Infrared radiation Ultraviolet radiation (b) Distinguish between a continuous spectrum and a line spectrum. (c) The thinning of the ozone layer increases the amount of UV-B radiation that reaches the Earth’s surface. Type of Radiation UV-A UV-B Wavelength / nm 320 - 380 290 - 320 Based on the information in the table above explain why UV-B rays more dangerous than UV-A. 4. A sample of iridium is analysed in a mass spectrometer. The first and last processes in mass sectrometry are vaporization and detection. (a)(i) State the names of the second and third processes in the order in which they occur in a mass spectrometer. (ii) Outline what occurs during the second process. (iii) State and explain which one of the following ions undergo the greatest deflection (under the same conditions in a mass spectrometer): 191 + Ir or 193Ir+ (b) The sample of iridium is found to have the following composition of stable isotopes: Isotope Relative abundance / % Ir – 191 37.1 Ir – 193 62.9 (i) Define the term relative atomic mass. (ii) Calculate the relative atomic mass of this sample of iridium, giving your answer to decmal places. (c) Iridium – 192 is a short-lived radioisotope used to treat cancer. Define the term radioisotope and name another radioisotope used in nuclear medcine. [(b)(ii) 192.26] 5. Describe the emission or line spectrum of gaseous hydrogen atoms and explain how this is related to the energy levels in the atom.