实验十苯妥英钠的制备
advertisement
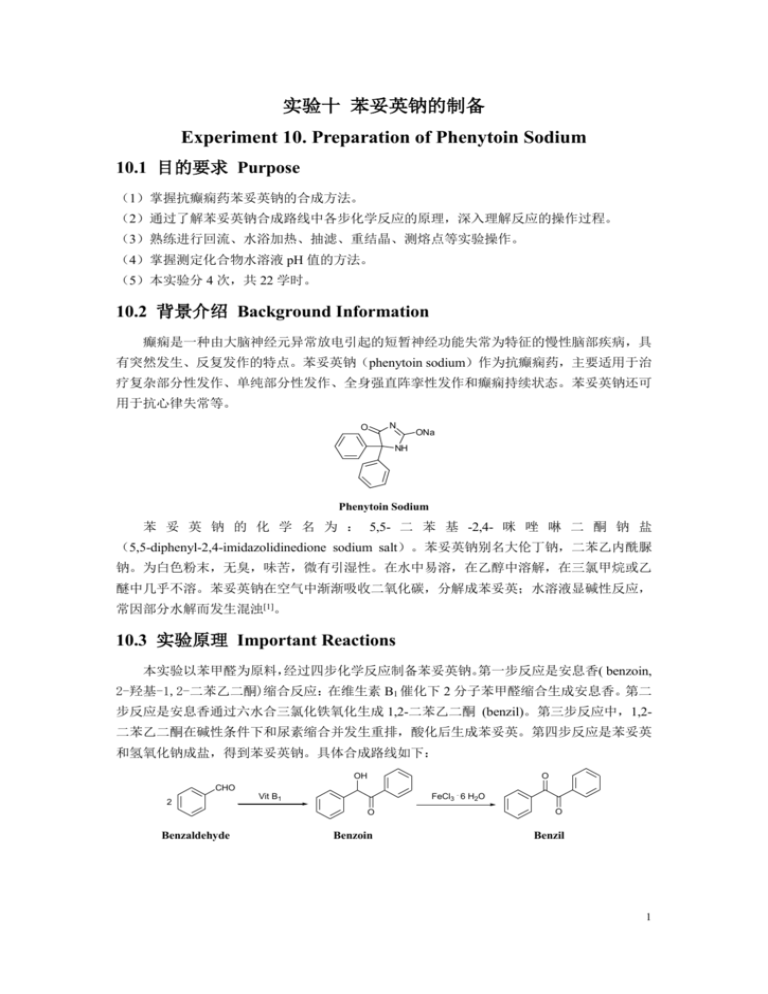
实验十 苯妥英钠的制备 Experiment 10. Preparation of Phenytoin Sodium 10.1 目的要求 Purpose (1)掌握抗癫痫药苯妥英钠的合成方法。 (2)通过了解苯妥英钠合成路线中各步化学反应的原理,深入理解反应的操作过程。 (3)熟练进行回流、水浴加热、抽滤、重结晶、测熔点等实验操作。 (4)掌握测定化合物水溶液 pH 值的方法。 (5)本实验分 4 次,共 22 学时。 10.2 背景介绍 Background Information 癫痫是一种由大脑神经元异常放电引起的短暂神经功能失常为特征的慢性脑部疾病,具 有突然发生、反复发作的特点。苯妥英钠(phenytoin sodium)作为抗癫痫药,主要适用于治 疗复杂部分性发作、单纯部分性发作、全身强直阵挛性发作和癫痫持续状态。苯妥英钠还可 用于抗心律失常等。 O N ONa NH Phenytoin Sodium 苯 妥 英 钠 的 化 学 名 为 : 5,5- 二 苯 基 -2,4- 咪 唑 啉 二 酮 钠 盐 (5,5-diphenyl-2,4-imidazolidinedione sodium salt)。苯妥英钠别名大伦丁钠,二苯乙内酰脲 钠。为白色粉末,无臭,味苦,微有引湿性。在水中易溶,在乙醇中溶解,在三氯甲烷或乙 醚中几乎不溶。苯妥英钠在空气中渐渐吸收二氧化碳,分解成苯妥英;水溶液显碱性反应, 常因部分水解而发生混浊[1]。 10.3 实验原理 Important Reactions 本实验以苯甲醛为原料, 经过四步化学反应制备苯妥英钠。 第一步反应是安息香( benzoin, 2-羟基-1,2-二苯乙二酮)缩合反应:在维生素 B1 催化下 2 分子苯甲醛缩合生成安息香。第二 步反应是安息香通过六水合三氯化铁氧化生成 1,2-二苯乙二酮 (benzil)。第三步反应中,1,2二苯乙二酮在碱性条件下和尿素缩合并发生重排,酸化后生成苯妥英。第四步反应是苯妥英 和氢氧化钠成盐,得到苯妥英钠。具体合成路线如下: O OH CHO 2 Benzaldehyde FeCl3 . 6 H2O Vit B1 O O Benzoin Benzil 1 O H N NH 1. NH2CONH2, NaOH O O N ONa NH NaOH 2. HCl Phenytoin Phenytoin sodium 在本实验的第一步,即安息香缩合反应中,除了以维生素 B1 催化的方法之外,还可以用 氰化钾[2]做催化剂。因为氰化钾是剧毒品,现已采用维生素 B1 代替氰化钾制备安息香。 在第二步氧化反应中,通常采用浓硝酸氧化。为了更适于学生实验的需要,用六水合三 氯化铁作为氧化剂,操作更为简单,且减少了实验室的污染。 10.4 实验操作 Experimental Procedures 10.4.1 2- 羟 基 -1,2- 二 苯 乙 二 酮 ( 安 息 香 ) 的 合 成 Synthesis of 2-Hydroxy-1,2-diphenylethanone ( Benzoin)(4 hours) OH CHO Vit B1 2 O Thiamine hydrochloride (1.75 g) is put into a 50-mL Erlenmeyer flask and distilled water (4 mL) is added to dissolve the solid. Then 95% alcohol (15 mL) is added into the solution. 2 mol/L aqueous solution of sodium hydroxide (5 mL) is added dropwise. The mixture is stirred at room temperature for 5 min. Finally benzaldehyde (10 mL) is added and the mixture is stood at room temperature for a week. The solid is collected on a Buchner filter by suction filtration. The solid is washed with a small amount of cold water. Then the solid is dried and the crude product of benzoin is obtained. The yield is calculated. The melting point is measured. Notes: (1) What is the crystal product when 1 mL of benzaldehyde and 5 mL of saturated solution of sodium bisulfite are mixed for a few minutes and then the mixture is cooled in an ice bath? (2) Benzaldehyde used in this reaction should be newly distilled. (3) The 2 mol/L NaOH aqueous solution should be prepared accurately. Questions: (1) What is the mechanism of this reaction? (2) Why the benzaldehyde should be newly distilled? (3) What is the catalytic mechanism of potassium cyanide? 10.4.2 1,2-二苯乙二酮的合成 Synthesis of 1,2-diphenylethanone (Benzil)(4 hours) O OH . FeCl3 6 H2O O O Benzoin (2.12 g), ferric chloride hexahydrate (9.0 g), and water (5 mL) are added into a 100-mL round-bottomed flask. Then two pieces of zeolite are added into the mixture. Then glacial 2 acetic acid (10 mL) is added after the flask is fitted with a refluxing condenser. The reaction mixture is heated to keep boiling for about 60 minutes. Then 50 mL of water is added into the flask. And the mixture is heated until the solution is boiling again. The residue in the flask is cooled. Some solid is precipitated. The solid is collected on a Buchner filter by suction filtration. Then the crude product of benzil is obtained. The crude product, a piece of zeolite, and 12 mL of ethanol are put into a pear-shaped flask. The flask is fitted with a refluxing condenser and is heated until the crude product is dissolved. Then the solution is cooled and some active charcoal is added into the solution. The mixture is heated to keep boiling for 10 minutes. Then the hot mixture is filtrated quickly on a Buchner filter by suction filtration. The filtrate is poured into a beaker quickly, cooled first at room temperature and then in an ice bath. When crystals of benzil are fully precipitated, collect the solid on a Buchner filter by suction filtration. The solid is washed with a small amount of cold mixture of ethanol and ether, dried and weighed. The yield of benzil is calculated and the melting point is measured[3]. Notes: (1) In this reaction, glacial acetic acid should be added into the round flask after the refluxing condenser has been attached. (2) Keep the contents of the flask boiling gently to avoid excessive evaporation of the solvent. (3) During the recrystallization process, we should filter the hot solution of benzil containing activated charcoal rapidly under vacuum. And the funnel and the leaching bottle should be preheated first. (4) The benzil should not be dried rapidly by washing it with ether. Questions: (1) Can concentrated nitric acid be used to oxidize benzoin in this experiment? (2) Why do we use ferric chloride hexahydrate as an oxidant instead of concentrated nitric acid in our experiment? 10.4.3 5,5- 二 苯 基 -2,4- 咪 唑 烷 二 酮 ( 苯 妥 英 ) 的 合 成 Synthesis of 5,5-Diphenyl-2,4-imidazolidinedione ( Phenytoin) (5 hours) O H N O 1. NH2CONH2, NaOH O O NH 2. HCl In this reaction, benzil is condensed with urea in the base condition. The starting materials, including the benzil which is synthesized in last reaction, urea, sodium hydroxide and alcohol, are mixed into a round flask according to the following scale. Benzil: urea: 15% NaOH: alcohol = 1 g : 0.57 g : 3.1 mL : 5 mL. And 2 pieces of zeolite are added into the flask. The flask is adapted with a refluxing condenser. Then the flask is heated on a water bath. The mixture in the flask will be boiling and kept for 2 hours. Then stop heating, the reactant is cooled at room temperature and is poured into a beaker containing the calculated amount of water (benzil: water = 1 g: 37 mL). The mixture in the beaker is stirred and stood at room temperature for about 15 minutes. Some solid 3 precipitation is filtered out. The filtrate is acidified with 15% hydrochloride acid until the pH value is about 4~5. Then the white solid is precipitated. The solid is filtrated on a Buchner filter by suction filtration and washed with a small amount of cold water. The solid is dried. Then the crude product of phenytoin is obtained and weighed. The yield of phenytoin is calculated and the melting point is measured[4]. Notes: (1) In this reaction, there are two filtration operations. During the first operation, the filtrate is taken and the solid by-product is filtered out. (2) During the reaction, calculated amount of 15% sodium hydroxide aqueous solution should be added to the bottom of the flask, not stick to the bottleneck. Questions: (1) What is the mechanism of the reaction? (2) What is the structure of the by-product of the reaction? 10.4.4 5,5- 二 苯 基 乙 内 酰 脲 钠 盐 ( 苯 妥 英 钠 ) 的 合 成 Synthesis of 5,5-Diphenyl-2,4-imidazolidinedione sodium salt ( Phenytoin Sodium)(3 hours) O H N O O NH NaOH N ONa NH The phenytoin synthesized in the last reaction is put into a flask. Then the calculated water (phenytoin: water = 1 g: 5 mL) is added. The flask is heated to 40℃ on a water bath. Then 15% sodium hydroxide aqueous solution is added slowly until the solid is all dissolved. Then some activated charcoal is added into the solution. The mixture is heated to 60℃ for 10 minutes and the solution is decolorized. Then the hot mixture is filtered quickly. The filtrate is stood at room temperature for about 20 minutes and cooled in cold water. Solid is precipitated and is filtered, washed with a small amount of cold water or a 1: 1 mixture of cold alcohol and acetyl ether. The solid is dried in vacuum and the phenytoin sodium is obtained. The weight of phenytoin sodium is measured and the yield is calculated. Notes: In this reaction, temperature of the contents in the flask should be controlled under 60℃. Questions: (1) Why the solid of phenytoin sodium is dried under vacuum? (2) Why do we use activated charcoal to decolorize the solution? 10.4.5 苯妥英钠溶液pH值的测定 The pH Value Measurement of the Solution of Phenytoin Sodium (6 hours) The pH value of phenytoin sodium solution is measured with the pH meter. First, the pH meter should be calibrated by 2 standard buffer solutions. (1) Preparation of the standard buffer solution of borax: borax (3.81 g) is weighed precisely and 4 put into a flask. Distilled water is added to dissolve it. Then the solution is diluted to 1000 mlL. (2) Preparation of the standard buffer solution of calcium hydroxide: at 25℃, prepare the saturated solution of calcium hydroxide with distilled water. Take the supernatant of the solution as the standard buffer. (3) Calibration of the pH meter: Calibrate the pH meter with the buffer solution of borax. For example, the pH value of borax buffer should be 9.18 at 25℃. When the borax buffer solution is used as the test solution, the pH value of pH meter should be adjusted to 9.18. Then the standard buffer solution of calcium hydroxide is used to calibrate the pH meter. At 25℃, the pH value of the standard buffer of calcium hydroxide is 12.45. Then measure the pH of the buffer and adujust the pH meter to show the value of 12.45. (4) Measurement Take 0.35 g of phenytoin sodium into a beaker, and add 10 mL distilled water to dissolve it. Then the pH value of the solution is measured using the pH meter for 2 times and take the average. 10.5 实验报告要求 Important Information About The Report One complete report should be written in the format of Appendix 2. In part of introduction and purpose, the background of the experiment, the chemical reactions should be included. Students should find the mechanisms of reactions of 10.4.1 and 10.4.3 by reference reading. In experimental part, the process and phenomenon of this experiment should be described elaborately. The experimental data should be recorded accurately such as the amounts of reagents, the molar ratios of the starting materials, and the form, weight, melting point and yield of the product in each reaction. In part of results & discussion, students should explain why your experiment worked or why not, and find out the reasons that support the success, lead to the failure or variations with the theory. Answers to the questions should be written in this part. In conclusion part, a brief description on the purpose, results and conclusions of the experiment should be given. 参考文献 References [1] 苯妥英钠. 见 国家药典委员会编.《中华人民共和国药典》二部. 北京:化学工业出版社, 2005,323. [2] Ray QB, Calvin AV, William EM. Benzoin, benzil and benzilic acid. In: Brewster Ray Q. Unitized Experiments in Organic Chemistry. 4thed. New York: Van Nostrand, 1977,425~429. [3] 杨仕豪, 李莉萍, 杨建文. 苯妥英钠的合成工艺改进. 中国医药工业杂志, 1995, 26 (1): 4~5. [4] 孙铁民. 苯妥英钠的合成. 见:孙铁民主编. 药物化学实验. 北京:中国医药科技出版社,2008,1~7. (北京大学药学院 邹晓民) 5








