Benzil Reduction Lab: Data & Analysis Sheet
advertisement

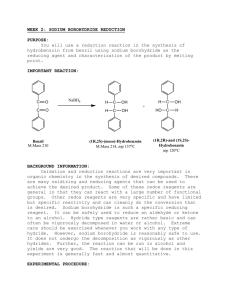
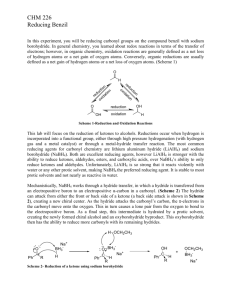
ORGANIC CHEMISTRY (II) LAB DATA & ANALYSIS SHEETS Reducing Benzil using Sodium Borohydride Student: _________________________ Date Performed: _______________ Partner: _________________________ Mass of crude benzil (~ 1 g): _______________ Mass of sodium borohydride (~ 0.2 g): _______________ Limiting reagent: _________________________ Theoretical yield (g): _______________ Mass of dry product: _______________ % yield: _______________ Physical description of product: ___________________________________________________ Calculations: Melting Points Experimental Dry product ____________ Product / (±)-benzoin ____________ Product / meso-hydrobenzoin ____________ Using your results, explain how the melting point and mixture melting point techniques can be used to identify your product. Cite literature sources (not the lab manual) for melting points of pure substances. Compare the IR spectrum of benzil with that of your product. Point out on the spectra the similarities and differences which support the identity or your product. benzil