Today's Experiment
advertisement

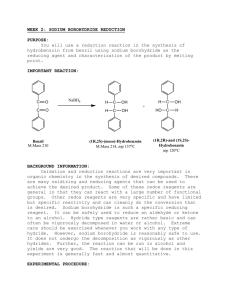
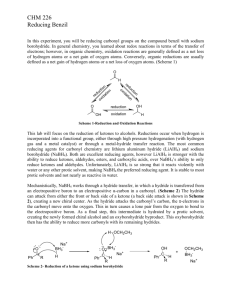
Stereoselective Reduction of Ketones with Sodium Borohydride: Making a Diol Experiment 15.1A: Microscale Reduction of Benzil Organic Chemistry Lab II, Spring 2009 Dr. Milkevitch Feb 23 & 25, 2009 Today’s Experiment Take a look at some chemistry of the C=O group Reduce the C=0 group to an alcohol Using the common reagent: – Sodium borohydride Purpose: – To illustrate the production of an alcohol by the reduction of a ketone – To use TLC and IR spectroscopy to characterize your results 1 Background Alcohols : – Useful class of organic compounds Readily available Cheap, relatively non-hazardous Can be converted to other compounds Reducing agents: _ – agents that add H: to compounds – Reduce carbonyl group to an alkoxide Subsequent protonation yields an alcohol Two Useful Hydride Agents Sodium Borohydride: NaBH4 Lithium Aluminum Hydride: LiAlH4 Called complex hydrides – Don’t have simple structure like NaH or LiH Today: Use NaBH4 – Safe to use – Highly selective reducing agent 2 Uses of Sodium Borohydride Reduces aldehydes to primary alcohols, ketones to secondary alcohols Very selective: – Only reactive towards aldehydes/ketones – Will not reduce carboxylic acids or esters Mechanism: Hydride Reduction of Carbonyl Group 3 Mechanism: Reduction of Benzil Step 1: Mechanism: Reduction of Benzil Step 2: Benzoin Step 3: Repeat of Steps 1, 2 •Reaction at the other C=O 4 Mechanism: Reduction of Benzil Final Product: hydrobenzoin But there is more than one product!!!!! Multiple Products Possible Remember, C=O is planar Hydride can attach carbon of C=O from above or below the plane Therefore, you can have different stereoisomers 5 What are the Multiple Products? •Hydrobenzoin has 2 chiral centers! •Have a meso compound •Pair of enantiomers Meso Pair of enantiomers Procedure Weigh out 200 mg of benzil into a 25 ml erlenmeyer flask Add 2 ml of 95% ethanol, swirl to mix Add 75 mg of sodium borohydride Let stand 10-15 min with occasional swirling Yellow color should disappear as benzil is reduced Add 2 ml of ddH2O slowly Heat to boiling, solution should be clear and colorless Filter off any insoluble impurities if necessary – Use a pasteur filter pipet – See me or Kt to make one Transfer the hot, clear solution to a clean 25 ml erlenmeyer flask, using a filter pipet Add an additional 2 ml of hot ddH2O Allow solution to crystallize undisturbed Cool in ice bath, vacuum filter – Wash with minimal cold ddH2O Weigh your product 6 Characterization Do a melting point determination TLC: dissolve 1-3 mg of your product in ethyl acetate – – – – EACH lab group member does a TLC plate!!! Dissolve 1-3 mg of (+/-)benzoin in ethyl acetate also Acquire a TLC plate (in oven) Spot the TLC plate with small amounts of your product and (+/-)benzoin – Run TLC plate in 9:1 methylene chloride/ethyl acetate This mobile phase is located in the hood – Stain with iodine (see Dr. M) Acquire an IR spectrum – Interpret your results Your Report Formal Report not required Complete experiment worksheet 7