Benzil Reduction: Stereochemistry Lab Manual
advertisement

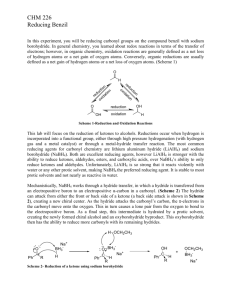
Stereochemical Analysis of Benzil Reduction Question: Is there diastereoselectivity in the reduction of benzil by sodium borohydride? In the first week of this lab, you will be designing your own procedure for the reduction of benzil with sodium borohydride to form hydrobenzoin . In week 2, you will make the acetonide of the diol so that you can use NMR to confirm the stereochemistry of the reduction. Background: NMR is often a valuable tool in determining the structure of compounds, even closely related stereoisomers. In this lab, you will reduce benzil to hydrobenzoin. There are a few different possibilities for the stereochemistry outcome of the reaction—the meso compound may be favored, the racemic mix may be formed, or a mixture of meso and racemic may form. Figure 1: Possible hydrobenzoin stereochemistries Sample: meso hydrobenzoin HO Sample: racemic hydrobenzoin HO OH OH +/- The NMR of these two samples will be very similar. It is often useful to make a derivative of the compound that will have a distinct NMR. In this case, making the acetonide (an acetal) of the hydrobenzoin may help us to distinguish between these samples using NMR. Procedure: Week 1: Reduction of benzil First, use SciFinder Scholar to find a primary literature source that gives a procedure for the reduction of acetophenone with sodium borohydride. Second, write a procedure for the reduction of benzil based on the literature you have found. You will need to answer these questions: What reagents will you use? How much? Which solvent, and how much? How long will the reaction proceed? What temperature? What is the quench/ work-up procedure? What is the isolation procedure? Do you need to do a further purification? If so, how can you tell? If so, what type of purification? Show your procedure to your AI before starting! Week 2: Acetonide formation Because this reaction is water sensitive, be sure all glassware is DRY before use! Put a 0.50 g sample of your hydrobenzoin and 150 mg of ferric chloride in a 25 mL roundbottom flask. Attach a reflux condenser, and add a stirbar Add 15 mL of anhydrous acetone to the reaction. Heat the reaction to boiling, and then allow to reflux for 20 minutes. Cool the solution and pour it into a separatory funnel containing 5 mL of 10% aqueous potassium carbonate. Swirl gently, then add an additional 25 mL of water. Extract your crude product from the aqueous mixture with two 10 mL portions of dichloromethane (which layer?) Combine the organic extracts and wash them with 10 mL of water. Dry the organic layer with sodium sulfate. Decant the dichloromethane from the drying agent into a small roundbottom flask, and remove the solvent to give an oily residue. Dissolve the crude oily residue in 10 mL of boiling petroleum ether (30-60 oC) and quickly hot filter through a Hirsch funnel into a small sidearm flask to remove insoluble unreacted diol. Continue to pull air through the sidearm flask until the petroleum ether is gone and an oil or soft solid remains. Weight the sidearm flask filled with product. Obtain a proton NMR. Clean the sidearm flask and weigh it. Dispose of waste as directed. Comment Questions: 1. How effective was the procedure you devised for the reduction? How would you change it if you were to perform the reaction again? 2. What is the mechanism of the acetonide formation? Based on your mechanism, explain these parts of the procedure: A. Why do all glassware and reagents have to be dry for this reaction? B. Why did you use iron trichloride in the reaction? C. What would have happened if you would have diluted the reaction with 10 mL of 10%HCl instead of 10% potassium bicarbonate? 3. Draw the structures of all the possible acetonide based on stereochemistry. Describe the differences you would expect in the 1H NMR spectra of these compounds. How is formation of the acetonide useful in determining the stereochemistry of the reactant? 4. Based on this data, what are your conclusions about the stereochemistry of your hydrobenzoin samples? What does this tell you about the diastereoselectivity of the reduction reaction? 5. Propose a mechanism of reduction that is sophisticated enough to account for your experimental observations.