Isotopes
advertisement

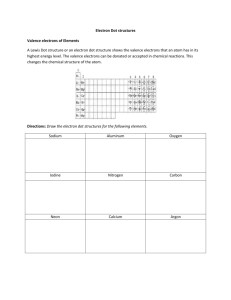
Isotopes • These are atoms of element with the same atomic number but different number of neutrons. • They therefore have different mass number. • Examples of isotopes: Hydrogen, Lithium, chlorine. Hydrogen has three isotopes e.g. 1 2 3 • H-1, H-2, H-3; 1H, 1H, 1H; Lithium has two isotopes • Li – 6, Li – 7; Chlorine has two isotopes • Cl -35, Cl – 37; 6 3Li, 7 35 37 17Cl, 17 3Li; Cl HOW COMPOUNDS ARE FORMED • A compound is a pure substance made up of two or more elements that are chemically combined. • Examples Water (H2O) & hydrogen peroxide (H2O2) • There are two types of compounds • Ionic compounds & molecular compounds IONIC COMPOUND • Ionic compounds are pure substances usually consisting of at least one metal and one non-metal. • example sodium chloride, NaCl • All ionic compound have high melting point • When they dissolve in water, the solution formed can conduct electricity. • The solution is called electrolyte Valence electrons Electrons occupy more than 99.99% of an atom’s volume. 1 • Electrons can move between energy levels. • The outermost shell that has electrons in it is called the valence shell. • Electrons in this shell are called valence electrons • Other shells containing electrons are called inner shells. • the electrons in them are called inner electrons. • The properties of elements are strongly affected by their valence electrons. • Other shells containing electrons are called inner shells. • the electrons in them are called inner electrons. • The properties of elements are strongly affected by their valence electrons. Some Elements in the periodic table showing valence Electrons 2 Forming Ionic Compounds • While combining, each atom changes into an ion. • Ions are formed when one or more electrons move from a metal atom over to a nonmetal atom. • a sodium atom loses an electron by giving it to a chlorine atom. • This produces a positive sodium ion and a negative chloride ion. • Positive and negative ions attract each other, so in an ionic compound, all the positive ions are attracted to all the negative ions. • A connection between atoms or ions is known as a bond. • The attractions between ions are called ionic bonds Ionic Compounds Names and Formulas of Common Compounds Common Names Drawing chalk Chemical Names calcium sulphate 3 antacid chalk calcium carbonate Naming Salts • In chemistry “salt” does not indicate which specific elements are found within a compound e.g. table salt Road salt sodium chloride. calcium chloride Clockwise from the lower left, the salts shown here are sodium chloride, ron(II) sulphate, iron(III) sulphate, copper(II) sulphate, and copper (II) carbonate Chemical Names and formula • Every compound has a chemical name and formula • For any element to form an ionic compound, it has to form an ion Ionic Charges Element Hydrogen lithium nitrogen oxygen Magnesium Aluminum Iron* Copper* Lead* Ion Charge 1+ 1+ 3– 2– 2+ 3+ 2+ or 3+ 1+ or 2+ 2+ or 4+ Ion Notation H+ Li+ N3– O2– Mg2+ Al3+ 2+ Fe or Fe3+ Cu+ or Cu2+ Pb2+ or Pb4+ 4 Ion Name hydrogen lithium nitride oxide magnesium aluminum iron(II) or iron(III) copper(I) or copper(II) lead(II) or lead(IV) Naming Ionic Compounds 1. Name the metal ion first. 2. If the element can form an ion in more than one way, include a Roman numeral to indicate the charge 3. Name the non-metal ion second. 4. When a non-metal becomes a negative ion, the ending of its name changes to “ide.” 5. The name for an ionic compound is a combination of the ion names of the elements. • The name of NaCl is, therefore, sodium chloride. • • Examples: • Positive Ion Negative Ion Formula Name 2+ • Mg O2– MgO • Ba2+ F– • K+ N3– • Pb2+ Cl- • Pb4+ N3- Molecular Compounds • When non-metals combine, a pure substance called a molecular compound is formed. • In molecular compounds, the atoms share electrons to form small groups, called molecules. • Molecular Compounds : can be solids, liquids, or gases at room temperature are usually good insulators but poor conductors of electricity have relatively low boiling points 5 Naming Molecular compounds • • • In naming molecular compounds, prefixes are used. Prefixes indicate the number of atoms present in the molecule E.g. Number of atoms Prefix 1 mono2 di3 tri4 tetra5 penta- Steps for naming molecular compounds 1. Examine the formula given N2O PBr3 CS2 2. Name the first element Nitrogen phosphorus Carbon 3. Name the second element and add –ide oxide bromide sulphide • Add prefixes indicating the number of atoms of each • Dinitrogen monoxide; phosphorus tribromide; carbon disulphide 6