Part IB
advertisement
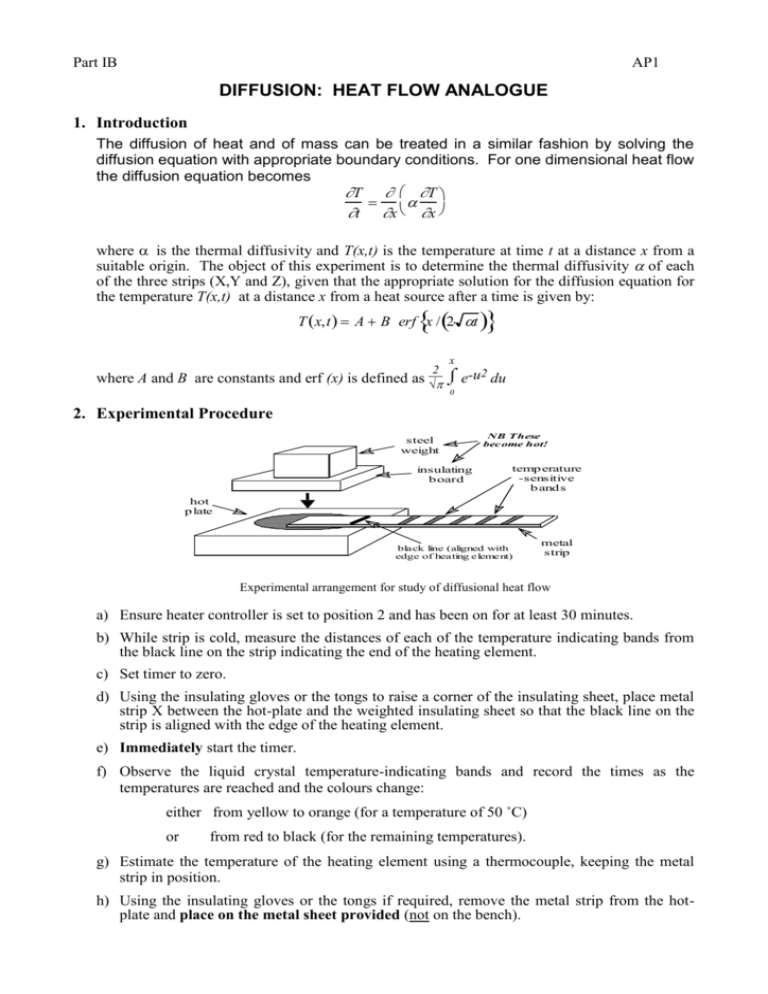
Part IB AP1 DIFFUSION: HEAT FLOW ANALOGUE 1. Introduction The diffusion of heat and of mass can be treated in a similar fashion by solving the diffusion equation with appropriate boundary conditions. For one dimensional heat flow the diffusion equation becomes T T t x x where is the thermal diffusivity and T(x,t) is the temperature at time t at a distance x from a suitable origin. The object of this experiment is to determine the thermal diffusivity of each of the three strips (X,Y and Z), given that the appropriate solution for the diffusion equation for the temperature T(x,t) at a distance x from a heat source after a time is given by: T x,t A B erf x / 2 t x where A and B are constants and erf (x) is defined as 2 √ e-u2 du 0 2. Experimental Procedure steel weight insulating b oard NB These become hot! temp erature -sensitive b and s hot p late black line (aligned with edge of heating element) metal strip Experimental arrangement for study of diffusional heat flow a) Ensure heater controller is set to position 2 and has been on for at least 30 minutes. b) While strip is cold, measure the distances of each of the temperature indicating bands from the black line on the strip indicating the end of the heating element. c) Set timer to zero. d) Using the insulating gloves or the tongs to raise a corner of the insulating sheet, place metal strip X between the hot-plate and the weighted insulating sheet so that the black line on the strip is aligned with the edge of the heating element. e) Immediately start the timer. f) Observe the liquid crystal temperature-indicating bands and record the times as the temperatures are reached and the colours change: either from yellow to orange (for a temperature of 50 ˚C) or from red to black (for the remaining temperatures). g) Estimate the temperature of the heating element using a thermocouple, keeping the metal strip in position. h) Using the insulating gloves or the tongs if required, remove the metal strip from the hotplate and place on the metal sheet provided (not on the bench). Part IB AP1/2 i) Carry out the analysis outlined in §3. j) Repeat the operation for both strips Y and Z. 3. Analysis of Data i) Plot a graph of distance versus √ time for each of the five temperatures on the liquid crystal temperature-indicating bands. Both axes on the graph should begin at zero. ii) By using suitable boundary conditions, derive expressions in terms of temperature for the constants A and B in the equation provided. Note that erf (0) = 0 and erf () = l. iii) Using the values of the constants obtained, determine the thermal diffusivity of the material from each of the gradients of the lines plotted. Values of the error function are given in the Data Book. Would you expect, and do you see, a systematic variation in ? 4. Discussion The metallographic mounts (X, Y and Z) are of polished sections of the strips prior to rolling. Examine the sections and discuss whether the relative values of thermal diffusivity can be explained in terms of the observed microstructures. Explain briefly whether or not values of i) diffusion coefficient and ii) electrical conductivity for the three materials would be expected to follow a similar pattern to the values of thermal diffusivity. For strip X describe and account for the colours observed in the section which had been heated. HDB/IB/00 Part IB AP2 SOLIDIFICATION AND FABRICATION 1. Introduction This practical has two purposes - the direct observation of solidification phenomena and an introduction to some of the casting techniques in industrial use. When a liquid metal or alloy is poured into a mould and allowed to solidify undisturbed, the grain structure of the resulting ingot usually consists of zones containing chill, columnar or equiaxed grains. The relative proportion of these zones varies with the cooling rate and the composition of the alloy. The first experiment is a study of how these zones develop, using a transparent model-system. The phenomenon observed is precipitation from a supersaturated solution, rather than a conventional solidification process, but many of the features of the grain structure are similar in the two situations. The microstructure of cast metal is profoundly affected by the morphology of the liquid/solid interface. In many cases, this is dendritic. The shape and size of individual dendrites, and the extent of the mushy zone composed of dendrites and surrounding liquid, is dependent on the atomic structure of the interface, solute redistribution and heat flow. The second experiment involves the study of various interfacial structures, again using transparent model-systems. Two materials will be studied. In one of these the entropy of fusion is relatively low, as in most metallic systems, giving rise to an interface which is rough on an atomic scale and hence to dendrites that are rounded. In the other, the entropy of fusion is higher, as in many non-metallic systems, leading to atomically smooth interfaces, a tendency for certain crystallographic planes to be preferentially exposed and hence the formation of dendrites with facets. In the last part of the practical, there will be an opportunity to look at castings produced using a variety of techniques. Details of the microstructure, surface finish and defects such as porosity and macrosegregation may be correlated with the solidification conditions during casting. The techniques to be described include sand casting, gravity die casting, pressure die casting, squeeze casting, investment casting and centrifugal casting. One objective is to identify the constraints imposed by each technique in terms of shape complexity, size, wall thickness, soundness, surface finish and cost. 2. Observation of Grain Structure Development (a) SAFETY GLASSES MUST BE WORN WHEN USING LIQUID NITROGEN. (b) DO NOT TOUCH ANY OBJECT THAT HAS BEEN COOLED IN LIQUID NITROGEN Three brass moulds with transparent sides are cooled by pouring liquid nitrogen into the dish in which the mould stands, but not into the mould itself. When frost has formed on the mould, clear the outside of the perspex window facing you by spraying with alcohol from a wash bottle, but do not get alcohol into the mould. Illuminate the mould from behind in order to see the structures being formed. Part IB AP2/2 Make up a saturated solution of ammonium chloride in a beaker by stirring in the crystals at 60˚C on a hot-plate. Continue until dissolution stops. Pour some of this solution into a cooled mould. Observe that many fine crystals are formed during initial contact with the mould wall (“big bang” nucleation) and that these become redistributed throughout the liquid. This often occurs during the casting of metals. Those in the bulk of the liquid may quickly remelt, depending on the pouring superheat. This is desirable since they may grow and block the feed of liquid metal needed to compensate for the freezing contraction. In the present experiment, such remelting is difficult because of the lower thermal conductivity of non-metallic systems. Depending on whether the solution was fully saturated, you may observe that many of the “big bang” crystallites survive and grow to form equiaxed grains. In any event, those which remained adjacent to the mould walls after pouring stay unmelted and form the chill zone. The remelting of the “big bang” crystallites is promoted by a high pouring superheat. This can be simulated by heating the saturated solution from 60˚C to 90˚C before pouring into the mould. You should then see the liquid clear quickly after pouring, as most of the precipitates are taken back into solution. The development of the columnar zone should then be clearly visible. In some cases, this will extend across the complete section of the casting. In practice, it might be arrested by the development of an equiaxed zone as a result of solid fragments surviving ahead of the advancing columnar grains. A common source of solid fragments to form the equiaxed zone is the free surface, where crystallisation is stimulated by heat loss. These surface crystals sediment down into the interior. You can promote this process by blowing gently on the free surface. Another mechanism by which equiaxed crystals form in castings is grain multiplication, for example by the detachment of dendrite arms at the advancing front as a result of mechanical and/or thermal disturbances. This cannot be readily promoted in the present experiment, since the growing crystals do not have the branched dendritic morphology which favours this. You may be able to promote grain multiplication by (gently!) tapping the windows. 3. Observation of Dendritic Structures 3.1 Dendritic Growth The interface remains planar during crystal growth from a pure melt with a positive temperature gradient in the direction of growth. Although a positive thermal gradient is usually present, the melt is never entirely pure. An impurity which partitions into the liquid leads to an accumulation of solute in the liquid adjacent to the growing crystal, thereby depressing its freezing point. There is then a larger driving force for solidification of liquid ahead of the interface than at the interface, even though the former is hotter. Such liquid is said to be “constitutionally” undercooled, to highlight the fact that it is its composition, rather than its temperature, which is responsible for its having a strong tendency to freeze. In this unstable situation, protrusions on the growth front grow rapidly into the supercooled liquid, giving familiar dendritic (from Greek for “tree”) structure. Part IB AP2/3 Constitutional undercooling can usually only be avoided at very slow growth rates. The details of the growth morphology tend to vary with the strength of the constitutional undercooling. If the effect is weak, then cellular structures are formed, composed of arrays of parallel prisms. As the strength of the undercooling increases, these cells start to develop side branches and also to exhibit a stronger tendency to grow along well-defined crystallographic directions (the so-called “easy growth” directions). For cubic metals, these are the <100> directions. The precise reasons why this occurs are still not entirely clear, but the effect is thought to be due to anisotropy of the atomic addition kinetics at the interface. (Non-metals, most of which have atomically flat interfaces, tend to exhibit this growth anisotropy over the complete range of growth conditions.) The reorientation to the nearest easy growth direction is often taken as marking the transition from a cell to a dendrite. Further changes occur as constitutional undercooling increases, with side arms forming and a highly branched morphology developing. A change in growth rate tends to have two separate effects. It may alter the degree of constitutional undercooling, and hence the dendrite morphology, it also affects the scale of the structure, with faster cooling giving rise to finer dendrite spacings. 3.2 Experimental Procedure view with microscope camphene or salol liquid glass slides sealed with glue around edge heater cooler Experimental arrangement for study of dendritic growth The set-up is shown in the figure. Specimens will have been left for some time beforehand on each apparatus, with heaters and coolers switched on, to reach thermal equilibrium. The liquid/solid interface should be approximately planar and be located somewhere around the centre of the glass slide in the viewing field of the microscope. Growth can be stimulated by perturbing the thermal field. The easiest method of doing this is to slide the specimen towards the cooler. (The specimen is simply resting on both the heater and the cooler.) This should cause the growth front to advance. The growth rate can be controlled by changing the distance the specimen is moved. A degree of fine control can be exercised by blowing gently on the specimen. Part IB AP2/4 Two types of specimen are provided. One is camphene (melts at 51˚C) and the other is salol (melts at 42˚C). In both cases, impurity content is such that constitutional undercooling is readily stimulated. Camphene forms dendrites in a similar manner to metals. This is because it has a similarly low entropy of fusion since the molecules can move from the liquid to the crystal in a number of alternative orientations. This is analogous to a (monomolecular) metal, the atoms of which do not need to rotate as they enter the crystal structure. A number of the features outlined above can be studied with this specimen. The breakdown of a planar front to cells, followed by reorientation to the easy growth directions and the development a branched dendritic structure can be observed. It is also possible to study the competitive growth between neighbouring grains which is responsible for the development of the columnar zone. It will be seen that the dendrites of a grain in which one of the easy growth directions is approximately parallel to the heat flow direction will grow faster than those of a less favourably oriented neighbour, which will gradually be excluded from further growth. The other specimen, salol, provides an analogue for the growth of faceted dendrites. It has a relatively high entropy of fusion, typical of materials with strong directional bonds and with molecular structures in which reorientation, as well as translation, are necessary as transfer takes place from liquid to solid. (Faceted dendrites can also arise with metallic phases, provided the entropy of fusion is high for some reason, e.g. Al dendrites in a tin-rich Sn-Al alloy. The entropy of fusion is high because the Al is so dilute in the melt, an unusual situation for a primary metallic phase.) The structures observed with the salol are often a little less obviously dendritic than the camphene, since the facets tend to dominate the appearance. Nevertheless, a plane front tends to break down to a dendritic structure in a similar manner as the camphene. The transition is more sluggish and reorientation is not observed, since growth only occurs in an easy direction. Both of these effects are consequences of the relatively high undercoolings needed for any interfacial advance. References 1. W.Kurz and D.J.Fisher, "Fundamentals of Solidification", Trans Tech., (1986) [Ng100] 2. www.msm.cam.ac.uk/phase-trans/dendrites.html 3. www.msm.cam.ac.uk/phase-trans/phase.field.models/movies2.html 4. www.msm.cam.ac.uk/phase-trans/phase.field.models/movies.html The last two references are computer generated or real movies of solidification, showing all of the features studied in this practical. You should feel free to download references 2-4 on to your own computers for future reference. HDB/IB/00 Part IB AP3 THE HARDENABILITY OF STEEL 1. Introduction Most heat treatments for steels begin by heating the specimen into the austenite phase field. The resulting austenite is then cooled continuously to room temperature. This is achieved by plunging the specimen into a bath of water or oil, or by removing it from the furnace to cool in air (“normalising”). If very slow cooling is required then the sample is left in the furnace which is switched off. The actual cooling rates may vary in different regions of the sample. These variations may be large since steels are relatively poor conductors of heat (thermal diffusivity of steel is about 10 -5 m2 s-1, of copper about ~10-4 m2 s-1). The properties of steels are sensitive to microstructure. It is useful to know how the microstructure develops in different parts of a specimen during heat treatment. For a given steel composition, a Continuous Cooling Transformation (CCT) diagram can be constructed from experimental data, allowing the microstructural development to be followed as a function of the cooling conditions. Fig.1 shows a CCT diagram for a eutectoid (Fe-0.8 wt %C) steel. Curves are plotted for the onset and completion of reactions to form pearlite, bainite and martensite. The former two have the “C” shape because the driving force is small at high temperatures whereas diffusion becomes sluggish at low temperatures. Martensitic transformation is represented by a line parallel to the time axis, since no diffusion is involved and because of the very high rate of growth, the fraction transformed depends only on the temperature. 900 Austenitising Temperature 800 Eutectoid Temperature Temperature (ÞC) 700 600 Pearlite 500 400 Bainite 300 Martens ite 200 100 Water Quench MARTENSITE 10 -1 Normalise Oil Quench FINE MARTENSITE/ BAINITE/ PEARLITE FINE PEARLITE 0 10 1 10 10 2 103 Furnace Cool COARSE PEARLITE 4 10 Time (s) Figure 1. Typical CCT diagram for a eutectoid steel. CCT diagrams are usually plotted with a linear temperature axis and a logarithmic time axis. A constant cooling rate therefore plots as having a continuously increasing gradient. In analysing real experimental results the true thermal history can be plotted even when the cooling rate is not constant. The dotted cooling line represents critical cooling conditions. Cooling faster than this avoids all transformations other than martensite. Since this will normally produce a specimen having the highest hardness, the critical cooling rate is a measure of the hardenability of the steel. The hardness generally decreases with decreasing cooling rate, even for microstructures without martensite. A steel with a high hardenability is one which has a low critical cooling rate, so that even slow cooling will lead to a martensitic structure. This has the advantage that hard material can be generated without the risk of “quench cracking” due to high thermal gradients associated with rapid cooling. On the other hand, it means that hard (and brittle) material may inadvertently be produced in finished artefacts, notably as a result of welding operations. 2. The Jominy End Quench Test The hardenability is measured by quenching one end of a hot bar (Fig.2). The bar is heated to the austenitising temperature, placed on a support and directionally cooled with a water jet. When cold, the specimen is sectioned and hardness measurements are made at intervals along its length. A wide variety of cooling rates and corresponding hardness data are revealed in a single and spectacular test. Part IB AP3/2 The hardenability may be represented by the critical cooling rate, or the critical distance along the bar at which the hardness (martensite content) starts to drop. It is useful to examine the microstructure and correlate it with the hardness as a function of position along the Jominy bar. Figure 2. Jominy end-quench specimen and corresponding hardness plot 3. Factors affecting Hardenability The alloying elements in steel have a big influence on hardenability. This is particularly so for diffusional transformations such as ferrite and pearlite, where the solutes not only influence the thermodynamic stability of the austenite but can slow the reactions by diffusion since their solubility in ferrite will be different from that in austenite. Displacive transformations such as bainite and martensite are less affected. Elements (Mn, Ni, C) which retard the transformation of austenite and hence shift the ‘C’ curves to longer times and vice versa (Co, Al). Hardenability is also affected by the austenite grain size. A finer grain size gives a larger number density of heterogeneous nucleation sites and hence reduces hardenability. 4. Tempering and Secondary Hardening Martensite in steel can be hard but brittle because of its excessive carbon content. Tempering involves a heat treatment which allows the carbon to precipitate as carbides (e.g. cementite). This, and the annealing of defects, causes the martensite to become softer but tougher. If the tempering temperature is sufficiently high (500 C) then substitutional elements such as Mo and Cr become mobile. Fine carbides such as Mo2C then precipitate at the expense of cementite and lead to secondary hardening. 5. Experimental Procedure The compositions of the steels studied are given in Table 1. Two sets of specimens with these compositions have already been quenched. One of these sets was tempered after quenching (Table II). There are therefore, six sections available for hardness tests along the length of the bars. Part IB AP3/3 Specimen Composition (wt %) Code C Mn Mo Ni Cr S1 0.31 - - - - S2 0.33 1.5 0.25 - - S3 0.31 - 0.5 3.3 0.8 Table I. Compositions of the three steels used for Jominy end quench testing. Treatment Times and Temperatures Code Austenitising Quenching Ageing O 1 hour @ 900˚C End Quenched - X 1 hour @ 900˚C End Quenched 30 mins. @ 650˚C Table II. Heat treatments applied to the three steels used for Jominy end quench testing. 5.2 Operations 5.2.1 The Jominy End Quench Test Do this test on any specimen, with the assistance of a demonstrator. 5.2.2 Vickers Hardness Measurement Measure hardness along the length of two Jominy specimens from the same alloy, one in the quenched (O) and the other in the quenched and tempered (X) condition . Results should be shared with other groups to compile hardness profiles for each of the specimens S1, S2 and S3. 5.2.3 Hardenability Assessment For S1(O), S2(O) and S3(O), establish the critical distance along the bar at which the hardness starts to fall below that of the fully martensitic structure. For low hardenability steels martensite is produced in a thin layer near the quenched surface. Rank the specimens in order of hardenability. For S1(O), S2(O) and S3(O), estimate the critical cooling rates using a simple analysis of the heat flow during quenching (see practical AP1). Assume that the specimen is initially at 900˚C and that the quench instantaneously brings one end to a temperature of 50˚C. The error function solution then allows the complete thermal history of the bar to be predicted. (thermal diffusivity 10-5 m2 s-1.) The transformation diagram for each steel is provided in Fig.4. For the distance from the quenched end of the bar found experimentally to be the limit of martensite formation, plot on the transformation diagram the predicted (error function) thermal history and see whether this does indeed just miss the nose of the diffusional transformation curve. Calculate the (constant) cooling rate which would also give a curve passing through this point. Comment on any discrepancies. Part IB AP3/4 5.2.4 Metallographic Examination Examine the microstructures of the mounted specimen S2(O) at several positions along the length of the bar. Many of the important features of steel microstructures are impossible to see using optical microscopy. You will not, for example, be able to see the carbides that form on tempering martensite. Transmission or scanning electron microscopy has to be used. Examples of such micrographs have been provided. Correlate these observations with the measured hardness profiles. Repeat for specimen S2(X). Explain your observations. Figure 4. Transformation diagrams for 3 steels having similar compositions to specimens S1-S3 References 1. R.W.K Honeycombe and H.K.D.H. Bhadeshia, “Steels”, 2nd edition, Edward Arnold (1995) [De88] 2. D.A. Porter & K.E. Easterling, “Phase Transformations in Metals & Alloys”, Chapters 5 and 6, Van Nostrand Rheinhold, (1981) [Ln30] 3. R.E. Reed-Hill, “Physical Metallurgical Principles”, Chapter 18, Van Nostrand Rheinhold, (1973) [A108] HDB/IB/00 Part IB AP4 EXAMPLES CLASS Start with Qu.1 or Qu.2, as directed. The class takes up the first 40 minutes of the practical period. 1. A zinc die casting is to be produced with a maximum section thickness of 8 mm. The casting must be ejected from the die 4 seconds after the liquid charge has been injected. The die remains at 100˚C throughout and the liquid is injected without superheat. Estimate the minimum heat transfer coefficient required at the die/casting interface if the casting is to be fully solid when ejected. Comment on the practical feasibility of achieving the required value. [For Zn, fusion temperature, Tf 420˚C, latent heat of fusion, H f 5 108 J m–3 , thermal conductivity, K 40 W m–1 K –1 ] 2. The carburization of steels is an important example of a surface-hardening process. Since the surface concentration of carbon is held constant as the carbon diffuses into the steel, the error function solution to the diffusion equation is applicable. In this question we solve this equation using the MATTER software. Open the MATTER module on Atomic Diffusion in metals and Alloys - Interstitial Diffusion, and turn to p.14. Assume that the initial concentration of carbon in the steel is 0.2 wt.%, and that the surface concentration is 0.8 wt.%. Set these values (under ‘Options’), and a temperature of close to 1100˚C. Select the plotting interval ‘small (smoother)’. Plot. (a) (b) (c) (d) What is the diffusion coefficient of carbon? After 1000 s of carburization what depth of material has a carbon content 0.6 wt.%? After 1000 s of carburization what depth of material has a carbon content 0.4 wt.%? A sample given this carburizing treatment is air-cooled to room temperature. Indicate, using sketches, how the microstructures near the surface of the sample and in the bulk would differ. [This examples class is followed, within the normal practical period, by an assessed practical lasting one hour. The assessed practical is based on AP1, AP2, AP3, and this examples class; it includes a small amount of practical work and some usage of the MATTER software. HDB/IB/00











