SOLIDIFICATION AND FABRICATION
advertisement

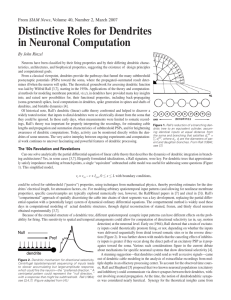
SOLIDIFICATION AND FABRICATION 1. Introduction This practical has two purposes - the direct observation of solidification phenomena and an introduction to some of the casting techniques in industrial use. When a liquid metal or alloy is poured into a mould and allowed to solidify undisturbed, the grain structure of the resulting ingot usually consists of zones containing chill, columnar or equiaxed grains. The relative proportion of these zones varies with the cooling rate and the composition of the alloy. The first experiment is a study of how these zones develop, using a transparent model-system. The phenomenon observed is precipitation from a supersaturated solution, rather than a conventional solidification process, but many of the features of the grain structure are similar in the two situations. The microstructure of cast metal is profoundly affected by the morphology of the liquid/solid interface. In many cases, this is dendritic. The shape and size of individual dendrites, and the extent of the mushy zone composed of dendrites and surrounding liquid, is dependent on the atomic structure of the interface, solute redistribution and heat flow. The second experiment involves the study of various interfacial structures, again using transparent model-systems. Two materials will be studied. In one of these the entropy of fusion is relatively low, as in most metallic systems, giving rise to an interface which is rough on an atomic scale and hence to dendrites that are rounded. In the other, the entropy of fusion is higher, as in many non-metallic systems, leading to atomically smooth interfaces, a tendency for certain crystallographic planes to be preferentially exposed and hence the formation of dendrites with facets. In the last part of the practical, there will be an opportunity to look at castings produced using a variety of techniques. Details of the microstructure, surface finish and defects such as porosity and macrosegregation may be correlated with the solidification conditions during casting. The techniques to be described include sand casting, gravity die casting, pressure die casting, squeeze casting, investment casting and centrifugal casting. One objective is to identify the constraints imposed by each technique in terms of shape complexity, size, wall thickness, soundness, surface finish and cost. 2. Observation of Grain Structure Development (a) SAFETY GLASSES MUST BE WORN WHEN USING LIQUID NITROGEN. (b) DO NOT TOUCH ANY OBJECT THAT HAS BEEN COOLED IN LIQUID NITROGEN Three brass moulds with transparent sides are cooled by pouring liquid nitrogen into the dish in which the mould stands, but not into the mould itself. When frost has formed on the mould, clear the outside of the perspex window facing you by spraying with alcohol from a wash bottle, but do not get alcohol into the mould. Illuminate the mould from behind in order to see the structures being formed. Part IB AP2/2 Make up a saturated solution of ammonium chloride in a beaker by stirring in the crystals at 60˚C on a hot-plate. Continue until dissolution stops. Pour some of this solution into a cooled mould. Observe that many fine crystals are formed during initial contact with the mould wall (“big bang” nucleation) and that these become redistributed throughout the liquid. This often occurs during the casting of metals. Those in the bulk of the liquid may quickly remelt, depending on the pouring superheat. This is desirable since they may grow and block the feed of liquid metal needed to compensate for the freezing contraction. In the present experiment, such remelting is difficult because of the lower thermal conductivity of non-metallic systems. Depending on whether the solution was fully saturated, you may observe that many of the “big bang” crystallites survive and grow to form equiaxed grains. In any event, those which remained adjacent to the mould walls after pouring stay unmelted and form the chill zone. The remelting of the “big bang” crystallites is promoted by a high pouring superheat. This can be simulated by heating the saturated solution from 60˚C to 90˚C before pouring into the mould. You should then see the liquid clear quickly after pouring, as most of the precipitates are taken back into solution. The development of the columnar zone should then be clearly visible. In some cases, this will extend across the complete section of the casting. In practice, it might be arrested by the development of an equiaxed zone as a result of solid fragments surviving ahead of the advancing columnar grains. A common source of solid fragments to form the equiaxed zone is the free surface, where crystallisation is stimulated by heat loss. These surface crystals sediment down into the interior. You can promote this process by blowing gently on the free surface. Another mechanism by which equiaxed crystals form in castings is grain multiplication, for example by the detachment of dendrite arms at the advancing front as a result of mechanical and/or thermal disturbances. This cannot be readily promoted in the present experiment, since the growing crystals do not have the branched dendritic morphology which favours this. You may be able to promote grain multiplication by (gently!) tapping the windows. 3. Observation of Dendritic Structures 3.1 Dendritic Growth The interface remains planar during crystal growth from a pure melt with a positive temperature gradient in the direction of growth. Although a positive thermal gradient is usually present, the melt is never entirely pure. An impurity which partitions into the liquid leads to an accumulation of solute in the liquid adjacent to the growing crystal, thereby depressing its freezing point. There is then a larger driving force for solidification of liquid ahead of the interface than at the interface, even though the former is hotter. Such liquid is said to be “constitutionally” undercooled, to highlight the fact that it is its composition, rather than its temperature, which is responsible for its having a strong tendency to freeze. In this unstable situation, protrusions on the growth front grow rapidly into the supercooled liquid, giving familiar dendritic (from Greek for “tree”) structure. Part IB AP2/3 Constitutional undercooling can usually only be avoided at very slow growth rates. The details of the growth morphology tend to vary with the strength of the constitutional undercooling. If the effect is weak, then cellular structures are formed, composed of arrays of parallel prisms. As the strength of the undercooling increases, these cells start to develop side branches and also to exhibit a stronger tendency to grow along well-defined crystallographic directions (the so-called “easy growth” directions). For cubic metals, these are the <100> directions. The precise reasons why this occurs are still not entirely clear, but the effect is thought to be due to anisotropy of the atomic addition kinetics at the interface. (Non-metals, most of which have atomically flat interfaces, tend to exhibit this growth anisotropy over the complete range of growth conditions.) The reorientation to the nearest easy growth direction is often taken as marking the transition from a cell to a dendrite. Further changes occur as constitutional undercooling increases, with side arms forming and a highly branched morphology developing. A change in growth rate tends to have two separate effects. It may alter the degree of constitutional undercooling, and hence the dendrite morphology, it also affects the scale of the structure, with faster cooling giving rise to finer dendrite spacings. 3.2 Experimental Procedure view with microscope camphene or salol liquid glass slides sealed with glue around edge heater cooler Experimental arrangement for study of dendritic growth The set-up is shown in the figure. Specimens will have been left for some time beforehand on each apparatus, with heaters and coolers switched on, to reach thermal equilibrium. The liquid/solid interface should be approximately planar and be located somewhere around the centre of the glass slide in the viewing field of the microscope. Growth can be stimulated by perturbing the thermal field. The easiest method of doing this is to slide the specimen towards the cooler. (The specimen is simply resting on both the heater and the cooler.) This should cause the growth front to advance. The growth rate can be controlled by changing the distance the specimen is moved. A degree of fine control can be exercised by blowing gently on the specimen. Part IB AP2/4 Two types of specimen are provided. One is camphene (melts at 51˚C) and the other is salol (melts at 42˚C). In both cases, impurity content is such that constitutional undercooling is readily stimulated. Camphene forms dendrites in a similar manner to metals. This is because it has a similarly low entropy of fusion since the molecules can move from the liquid to the crystal in a number of alternative orientations. This is analogous to a (monomolecular) metal, the atoms of which do not need to rotate as they enter the crystal structure. A number of the features outlined above can be studied with this specimen. The breakdown of a planar front to cells, followed by reorientation to the easy growth directions and the development a branched dendritic structure can be observed. It is also possible to study the competitive growth between neighbouring grains which is responsible for the development of the columnar zone. It will be seen that the dendrites of a grain in which one of the easy growth directions is approximately parallel to the heat flow direction will grow faster than those of a less favourably oriented neighbour, which will gradually be excluded from further growth. The other specimen, salol, provides an analogue for the growth of faceted dendrites. It has a relatively high entropy of fusion, typical of materials with strong directional bonds and with molecular structures in which reorientation, as well as translation, are necessary as transfer takes place from liquid to solid. (Faceted dendrites can also arise with metallic phases, provided the entropy of fusion is high for some reason, e.g. Al dendrites in a tin-rich Sn-Al alloy. The entropy of fusion is high because the Al is so dilute in the melt, an unusual situation for a primary metallic phase.) The structures observed with the salol are often a little less obviously dendritic than the camphene, since the facets tend to dominate the appearance. Nevertheless, a plane front tends to break down to a dendritic structure in a similar manner as the camphene. The transition is more sluggish and reorientation is not observed, since growth only occurs in an easy direction. Both of these effects are consequences of the relatively high undercoolings needed for any interfacial advance. References 1. W.Kurz and D.J.Fisher, "Fundamentals of Solidification", Trans Tech., (1986) [Ng100] 2. www.msm.cam.ac.uk/phase-trans/dendrites.html 3. www.msm.cam.ac.uk/phase-trans/phase.field.models/movies2.html 4. www.msm.cam.ac.uk/phase-trans/phase.field.models/movies.html The last two references are computer generated or real movies of solidification, showing all of the features studied in this practical. You should feel free to download references 2-4 on to your own computers for future reference. HDB/IB/00