Chemistry Homework: Balancing Equations & Conservation of Mass
advertisement

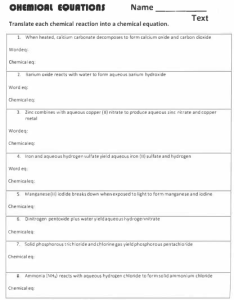
Name ______________________________________ Date ____________ Class _______________________ Modern Chemistry • CHAPTER 8 HOMEWORK 8-1 (pp. 240-255) Translate the following word equations into formula equations. BE SURE TO USE THE CHARGES OF THE IONS TO WRITE CORRECT FORMULAS. Be sure to include the states of the reactants and the products. Determine the sum of the masses of the reactants and the sum of the masses of the products. Show your work. Put yes or no into the blank indicating whether or not the equation as it is written follows the law of conservation of mass. _____1. Solid magnesium ribbon and aqueous hydrochloric acid react to form aqueous magnesium chloride and hydrogen gas. _____2. Solid aluminum carbide reacts with water to form methane gas and solid aluminum hydroxide. _____3. Solid calcium metal reacts with water to form aqueous calcium hydroxide and hydrogen gas. _____4. Nitrogen dioxide gas reacts with water to from aqueous nitric acid and nitrogen monoxide gas. _____5. Potassium chlorate solid decomposes when heated and forms potassium chloride solid and oxygen gas. _____6. When aqueous solutions of sulfuric acid and barium chloride are mixed, barium sulfate precipitates from an aqueous solution of hydrochloric acid. Name ______________________________________ Date ____________ Class _______________________ Modern Chemistry • CHAPTER 8 HOMEWORK 8-1 (pp. 240-255) _____7. Solid zinc oxide is made by reacting solid zinc sulfide with oxygen gas. The other product is sulfur dioxide gas. _____8. Aqueous silver nitrate reacts with aqueous sodium chloride to yield silver chloride as a precipitate and sodium nitrate in aqueous solution.