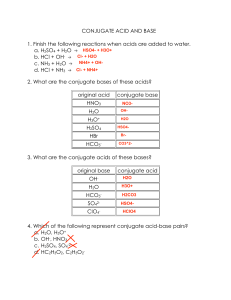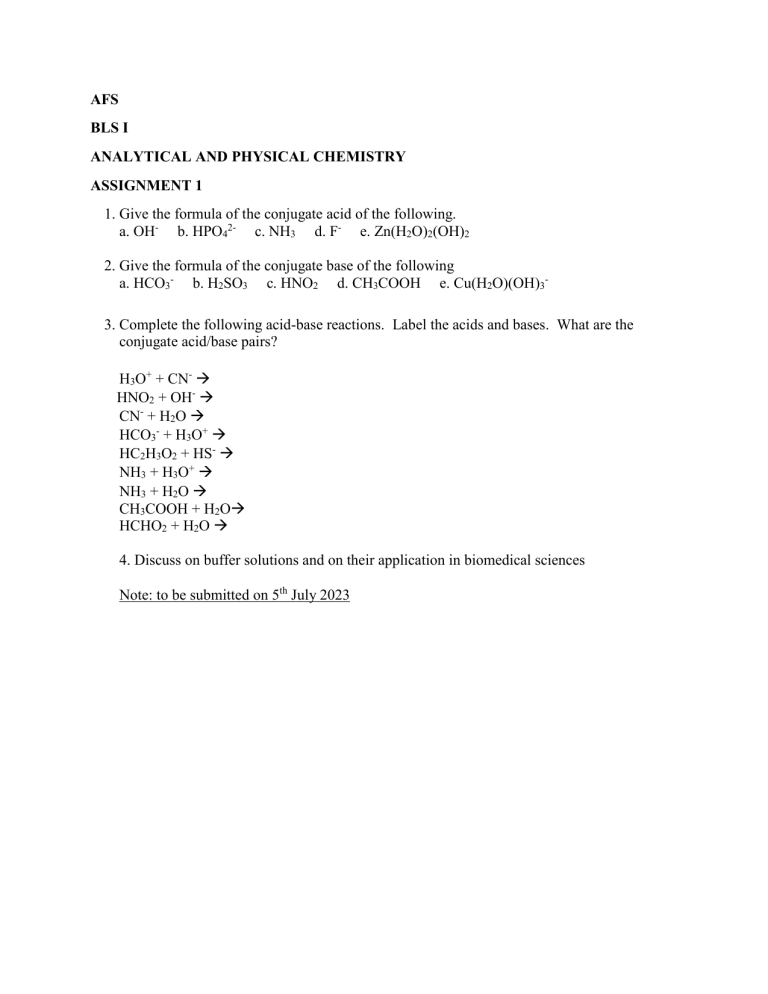
AFS BLS I ANALYTICAL AND PHYSICAL CHEMISTRY ASSIGNMENT 1 1. Give the formula of the conjugate acid of the following. a. OH- b. HPO42- c. NH3 d. F- e. Zn(H2O)2(OH)2 2. Give the formula of the conjugate base of the following a. HCO3- b. H2SO3 c. HNO2 d. CH3COOH e. Cu(H2O)(OH)33. Complete the following acid-base reactions. Label the acids and bases. What are the conjugate acid/base pairs? H3O+ + CN- HNO2 + OH- CN- + H2O HCO3- + H3O+ HC2H3O2 + HS- NH3 + H3O+ NH3 + H2O CH3COOH + H2O HCHO2 + H2O 4. Discuss on buffer solutions and on their application in biomedical sciences Note: to be submitted on 5th July 2023