Molar Mass Practice Worksheet
advertisement
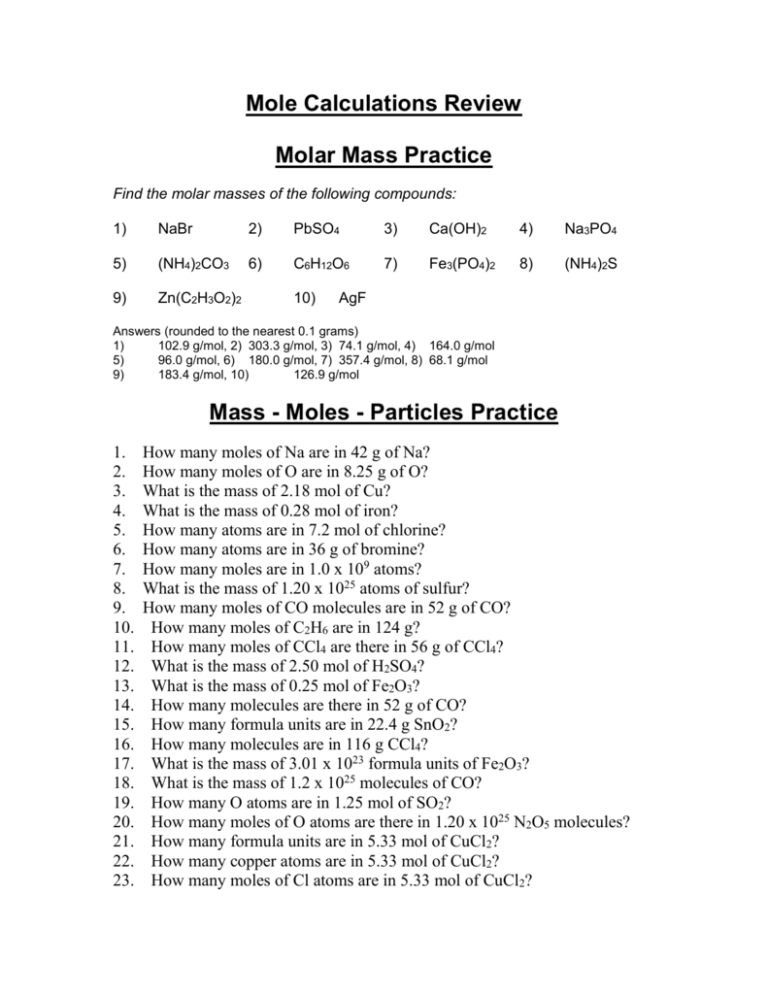
Mole Calculations Review Molar Mass Practice Find the molar masses of the following compounds: 1) NaBr 2) PbSO4 3) Ca(OH)2 4) Na3PO4 5) (NH4)2CO3 6) C6H12O6 7) Fe3(PO4)2 8) (NH4)2S 9) Zn(C2H3O2)2 10) AgF Answers (rounded to the nearest 0.1 grams) 1) 102.9 g/mol, 2) 303.3 g/mol, 3) 74.1 g/mol, 4) 164.0 g/mol 5) 96.0 g/mol, 6) 180.0 g/mol, 7) 357.4 g/mol, 8) 68.1 g/mol 9) 183.4 g/mol, 10) 126.9 g/mol Mass - Moles - Particles Practice 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. How many moles of Na are in 42 g of Na? How many moles of O are in 8.25 g of O? What is the mass of 2.18 mol of Cu? What is the mass of 0.28 mol of iron? How many atoms are in 7.2 mol of chlorine? How many atoms are in 36 g of bromine? How many moles are in 1.0 x 109 atoms? What is the mass of 1.20 x 1025 atoms of sulfur? How many moles of CO molecules are in 52 g of CO? How many moles of C2H6 are in 124 g? How many moles of CCl4 are there in 56 g of CCl4? What is the mass of 2.50 mol of H2SO4? What is the mass of 0.25 mol of Fe2O3? How many molecules are there in 52 g of CO? How many formula units are in 22.4 g SnO2? How many molecules are in 116 g CCl4? What is the mass of 3.01 x 1023 formula units of Fe2O3? What is the mass of 1.2 x 1025 molecules of CO? How many O atoms are in 1.25 mol of SO2? How many moles of O atoms are there in 1.20 x 1025 N2O5 molecules? How many formula units are in 5.33 mol of CuCl2? How many copper atoms are in 5.33 mol of CuCl2? How many moles of Cl atoms are in 5.33 mol of CuCl2? 24. How many moles of CuCl2 contain 1.2 x 1023 atoms of Cl? 25. How many O atoms are in 3.15 mol of SnO2? 26. How many H atoms are in 17.5 g (NH4)2C2O4? Answers 1. 6. 12. 16. 20. 24. , 2.7 x 10 Br atoms, 7. 1.7 x 10 mol, 8. 639 g S, 9. 1.9 mol, 10. 4.12 mol, 11. 0.36 mol, 245 g, 13. 40. g, 14. 1.1 x 1024 molecules, 15. 8.95 x 1022 formula units, 4.54 x 1023 molecules, 17. 79.9 g Fe2O3, 18. 5.6 x 102 g CO, 19. 1.51 x 1024 O atoms, 99.7 mol O, 21. 3.21 x 1024 formula units, 22. 3.21 x 1024 Cu atoms, 23. 10.7 mol of Cl atoms, 0.10 mol CuCl2, 25. 3.79 x 1024 O atoms, 26. 6.79 x 1023 H atoms 1.8 mol Na, 2. 23 0.516 mol O, 3. 139 g Cu, 4. 16 g Fe, 5. 4.3 x 1024 Cl atoms -15 Percent Composition and Molecular Formula Practice 1. What’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? 2. If the molar mass of the compound in problem 1 is 110 grams/mole, what’s the molecular formula? 3. What’s the empirical formula of a molecule containing 18.7% lithium, 16.3% carbon, and 65.0% oxygen? 4. If the molar mass of the compound in problem 3 is 73.8 grams/mole, what’s the molecular formula? Write the molecular formulas of the following compounds: 5. A compound with an empirical formula of C2H4O and a molar mass of 88 grams per mole. 6. A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole. 7. A compound with an empirical formula of CFBrO and a molar mass of 254.7 grams per mole. 8. A compound with an empirical formula of C2H8N and a molar mass of 46 grams per mole. Answer the following questions: 9. The percentage composition of acetic acid is found to be 39.9% C, 6.7% H, and 53.4% O. Determine the empirical formula of acetic acid. 10. The molar mass for question #9 was determined by experiment to be 60.0 g/mol. What is the molecular formula? 11. Aniline, a starting material for urethane plastic foams, consists of C, H, and N. Combustion of such compounds yields CO2, H2O, and N2 as products. If the combustion of 9.71 g of aniline yields 6.63 g H2O and 1.46 g N2, what is its empirical formula? 12. The molar mass of aniline is 93 g/mol. What is its molecular formula? 13. Calculate the mass percent of carbon, nitrogen and oxygen in acetamide, C2H5NO. 14. A 50.51 g sample of a compound made from phosphorus and chlorine is decomposed. Analysis of the products showed that 11.39 g of phosphorus atoms were produced. What is the empirical formula of the compound? 15. When 2.5000 g of an oxide of mercury, (HgxOy) is decomposed into the elements by heating, 2.405 g of mercury are produced. Calculate the empirical formula. 16. The compound benzamide has the following percent composition. What is the empirical formula? C = 69.40 % H= 5.825 % O = 13.21 % N= 11.57 % 17. A component of protein called serine has an approximate molar mass of 100 g/mole. If the percent composition is as follows, what is the empirical and molecular formula of serine? C = 34.95 % H= 6.844 % O = 46.56 % N= 13.59 % Percent Composition and Molecular Formula Answers 1. C3H3O mass = 55 g/mole, 2. C6H6O2, 3. Li2CO3, 4. Li2CO3, 5. C4H8O2, 6. C8H8O2, 7. C2F2Br2O2, 8. C2H8N, 9. CH2O, 10. C2H4O2, 11. C6H7N, 12. C6H7N, 13. %C 40.668 %H 8.533 %N 23.713 %O 27.086, 14. PCl3, 15. Hg2O, 16. C7H7NO, 17. C3H7NO3 empirical formula C3H7NO3 molecular formula Extra Practice 1) 2) 3) 4) 5) 6) 7) 8) Find the molar mass of Mg3(PO4)2. How many moles are in 17.7 g of KMnO4? How many atoms of O are in 17.7 g Ba(NO3)2? How many molecules are in 26.9 g of C12H22O11? How many moles are 19.8 x 1024 molecules C6H12O6? What is the mass of 19.8 x 1024 molecules C12H22O11? Find the percentage composition of K2Cr2O7. A compound has a percentage composition of 40.01% C, 6.69 % H, and 53.30% O. Its molar mass is 179.9 g/mol. What is its empirical formula? What is its molecular formula? 9) What is the empirical formula for a compound if a 83.7 g sample contains 44.28g Al and 39.42 g O? 10) A 1.640 g sample of radium metal was heated to produce 1.755 g of radium oxide. What is the empirical formula? 11) How many H atoms are in 72.5 g of C3H8O? 12) 1.77 x 1028 atoms Cu = ___ mole Cu = ___ grams Cu 13) 1.99 x 1025 molecules of H2O = ___ g H2O






