CH. 10 KEY
advertisement
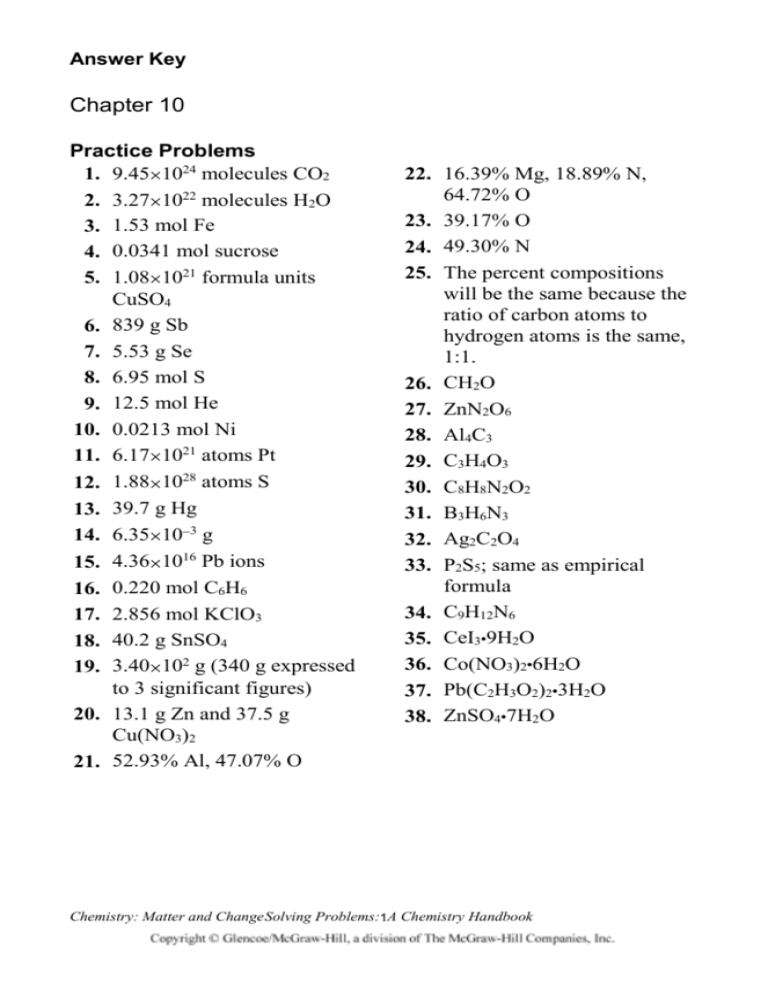
Answer Key Chapter 10 Practice Problems 1. 9.451024 molecules CO2 2. 3.271022 molecules H2O 3. 1.53 mol Fe 4. 0.0341 mol sucrose 5. 1.081021 formula units CuSO4 6. 839 g Sb 7. 5.53 g Se 8. 6.95 mol S 9. 12.5 mol He 10. 0.0213 mol Ni 11. 6.171021 atoms Pt 12. 1.881028 atoms S 13. 39.7 g Hg 14. 6.35103 g 15. 4.361016 Pb ions 16. 0.220 mol C6H6 17. 2.856 mol KClO3 18. 40.2 g SnSO4 19. 3.40102 g (340 g expressed to 3 significant figures) 20. 13.1 g Zn and 37.5 g Cu(NO3)2 21. 52.93% Al, 47.07% O 22. 16.39% Mg, 18.89% N, 64.72% O 23. 39.17% O 24. 49.30% N 25. The percent compositions will be the same because the ratio of carbon atoms to hydrogen atoms is the same, 1:1. 26. CH2O 27. ZnN2O6 28. Al4C3 29. C3H4O3 30. C8H8N2O2 31. B3H6N3 32. Ag2C2O4 33. P2S5; same as empirical formula 34. C9H12N6 35. CeI39H2O 36. Co(NO3)26H2O 37. Pb(C2H3O2)23H2O 38. ZnSO47H2O Chemistry: Matter and Change Solving Problems:1A Chemistry Handbook Answer Key (continued) Chapter 10 Review 39. One mole of a substance consists of 6.021023 particles of that substance. The mass in grams of a mole of a substance is numerically equal to the sum of the atomic masses of the atoms that make up the particles of that substance. 40. The quantities of reactants and products in a chemical equation are given as numbers of particles or moles of particles, not as masses. 41. The molar mass of a compound in grams is numerically equal to the sum of the atomic masses of the atoms in a representative particle of the compound. 42. 28.014 g, which is twice the atomic mass expressed in grams 43. Yes; you would expect the compositions to be the same. A compound is a substance, and the law of definite proportions states that the composition of a substance is the same wherever the substance is found. 44. The molecular formula will be a whole-number multiple of the empirical formula but could also be the same as the empirical formula. 45. The six water molecules are incorporated in the crystal structure but are separate from the compound. The formula with water molecules shown separately better represents the composition of the compound. Chemistry: Matter and Change Solving16Problems: A Chemistry Handbook