Photoelectric Effect Lesson Plan: Physics, Grade 12
advertisement

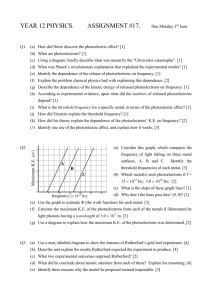
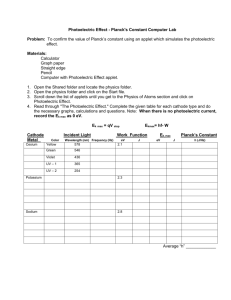
Author Bruce Dalzell Title Photoelectric Effect Grade Level 12 Subject Area Physics Overview of unit/lessons/activities (assumptions of prior knowledge/learning) Unit 7: Waves and Modern Physics Chapter 18: Early Quantum Theory Topic: 18.1 The Particle Nature of Light: The Photoelectric Effect Overview Students begin to examine the differences, similarities, and interactions between particles and waves. The photoelectric effect was a significant early demonstration of the quantum nature of light. The concept of threshold frequency and work function for metals will be examined as well as the effects of changing the battery voltage difference and the frequency and intensity of light on the electron velocity and resulting current. Activities 1. A set of five mini labs using the Photoelectric Effect Simulation - PhET Large / Small Group Practice / Discovery Learning (using Photoelectric Effect Simulation - PhET) - Individual Data Collection and Mathematical / Graphical Analysis of Results (PhET / DataStudio – work function, threshold frequency) Reinforcement Activity (PhET – work function) Individual Concluding Activity (PhET – unknown metal) 2. Teacher-centered instruction and large group discussion of results Assumptions / Prior Learning In Physics 11, students have studied particle and wave behaviour having completed Unit 4: Waves. The Waves unit included the Universal Wave Equation (c = f λ) and Planck’s equation (E = hf ) and kinetic energy (EK= ½mv2) Most Physics 12 students have successfully completed Chemistry 11 and understand the atomic theory and ionization energy. Correlations to ICT and curriculum outcomes ICT OUTCOMES TEN TO TWELVE BOC 12.1 use a wide variety of technology, demonstrate a clear understanding of technological applications, and consistently apply appropriate technology to solve curriculum problems BOC 12.2 demonstrate an ability to assess the application of technology to solve problems, particularly to evaluate significant effects which estimations, program flaws and human error have on a given solution PTS 12.5 create electronic charts, tables and graphs; and design, create and manipulate spreadsheets and databases, as part of the process of collecting, analyzing, and displaying data independently CT 12.3 design and create electronic documents to accomplish curricular tasks RPSD 12.1 select appropriate devices and software to collect data, solve problems and note patterns; to make logical decisions and draw conclusions; and to present results, with general supervision PHYSICS 12 OUTCOMES 115-7 explain how scientific knowledge evolves as new evidence comes to light and as laws and theories are tested and subsequently restricted, revised, or replaced 115-3 explain how a photon momentum revolutionized thinking in the scientific community 213-6 use library and electronic research tools to collect information on a given topic 327-9 describe how the quantum energy concept explains blackbody radiation and the photoelectric effect 327-10 explain qualitatively and apply the formula for the photoelectric effect Projected timeline for preparation and for carrying out activities 1. Teacher-centered instruction (TCI): Background Concepts and Introduction of the Photoelectric Effect Large Group Practice / Discovery Learning: Relating Frequency and Intensity to Current (Photoelectric Effect Simulation - PhET) 2. TCI: The Effects of Light Intensity and Frequency and The Experimental Work of Lenard and the Concept of Stopping Potential 3. Small Group Practice / Discovery Learning: Determining Stopping Potential (using Photoelectric Effect Simulation - PhET) 4. TCI: Einstein, Millikan, Work Function and Threshold Frequency 5. Individual Data Collection and Mathematical and Graphical Analysis of Results: Determination of Work Function and Threshold Frequency for a Specific Metal (using Photoelectric Effect Simulation - PhET) 6. Reinforcement Activity : Verification of the Work Function of a Metal from Simulation Data 7. Individual Concluding Activity: Identification of an Unknown Metal by from its Work Function and Threshold Frequency (using Photoelectric Effect Simulation - PhET) Equipment Requirements: Data projector and screen – for teacher demonstration of how to use simulation software (computers, software, etc) Computer Lab / COW (internet access) – for pairs of students to play/experiment, collect, store, and email data between home and school Web Site: http://phet.colorado.edu/new/simulations/sims.php?sim=Photoelectric_Effect Software: Data Studio (for graphical analysis) Teaching materials provided (Blacklines, worksheets, templates, teacher materials) Text: Physics (2003). Chapter 18 Early Quantum Theory: The photoelectric effect. McGraw-Hill Ryerson: Toronto. pp. 843-853. Files: SIMULATION EXPERIMENTS MiniLab 1: Relating Frequency and Intensity to Current MiniLab 2: Determining Stopping Potential Figure 1: Graph of Stopping Potential versus Frequency for Sodium Metal MiniLab 3: Determination of Work Function and Threshold Frequency for a Specific Metal Lab 4: Verification of the Work Function of a Metal from Simulation Data Lab 5: Identification of an Unknown Metal by from its Work Function and Threshold Frequency NOTES Introduction of the Photoelectric Effect The Effects of Light Intensity and Frequency The Experimental Work of Lenard and the Concept of Stopping Potential Einstein, Millikan, Work Function and Threshold Frequency Resources available for teacher/student use (websites, references, etc) SIMULATION SITE Photoelectric Effect Simulation - PhET http://phet.colorado.edu/new/simulations/sims.php?sim=Photoelectric_Effect ADDITIONAL LESSON PLANS FROM PHET Intro to Photoelectric Effect Interactive Lecture S. McKagan Demo Photoelectric effect J. Bourne Lab Photoelectric Effect C. Miller Lab Photoelectric Effect Activity D. Collins Lab The Photoelectric Effect A. Sokolowski Lab Understanding the Photoelectric Effect S. McKagan HW RELATED WEB SITES Physics 24/7: Physics Tutorial: Photoelectric Effect and Garage Door Sensors http://www.physics247.com/physics-tutorial/photoelectric-effect.shtml Robert B. Friedman & Rick Kessler - The Photoelectric Effect & Its Applications http://cfcpwork.uchicago.edu/kicp-projects/nsta/2007/pdf/nsta_2007-photoeleclab.pdf STEAMING VIDEO A Photoelectric Effect Experiment (Windows Media Audio/Video file) Detailed instructions for each activity or lesson (teacher notes, activity information, learning strategies, teacher role, student roles) Unit 7 – Waves and Modern Physics Chapter 18 Early Quantum Theory Photoelectric Effect 1. CLASS ONE A. TEACHER-CENTERED INSTRUCTION 18.1 THE PARTICLE NATURE OF LIGHT AND EARLY QUANTUM THEORY Classical physics, Maxwell, electromagnetic radiation, universal wave equation Universal Wave Equation (c = f λ) and Planck’s equation (E = hf ) and kinetic energy (EK= ½mv2) Atomic theory and ionization energy 2. CLASS TWO A. TEACHER-CENTERED INSTRUCTION INTRODUCTION OF THE PHOTOELECTRIC EFFECT (in the simplest of terms) B. LARGE GROUP PRACTICE / DISCOVERY LEARNING (using Photoelectric Effect Simulation) LAB 1: RELATING FREQUENCY AND INTENSITY TO CURRENT 3. CLASS THREE A. GROUP DISCUSSION OF RESULTS:LAB 1: RELATING FREQUENCY AND INTENSITY TO CURRENT B. TEACHER-CENTERED INSTRUCTION THE EFFECTS OF LIGHT INTENSITY AND FREQUENCY C. TEACHER-CENTERED INSTRUCTION THE EXPERIMENTAL WORK OF LENARD AND THE CONCEPT OF STOPPING POTENTIAL B. CLASS THREE A. SMALL GROUP PRACTICE / DISCOVERY LEARNING (using Photoelectric Effect Simulation) LAB 2: DETERMINING STOPPING POTENTIAL B. HOMEWORK ASSIGNMENT Construct a graph of Stopping Potential versus Frequency (use graphing calculators or Data Studio – Enter Data) o Determine the slope of the line of best fit in V∙s and in J∙s (1 eV = 1.6× 10−19 J) o Determine the x-intercept and the y-intercept (include range of uncertainty) C. CLASS FOUR A. GROUP DISCUSSION OF RESULTS FROM LAB 2: DETERMINING STOPPING POTENTIAL B. TEACHER-CENTERED INSTRUCTION EINSTEIN, MILLIKAN, WORK FUNCTION AND THRESHOLD FREQUENCY D. CLASS FIVE A. INDIVIDUAL DATA COLLECTION AND MATHEMATICAL AND GRAPHICAL ANALYSIS OF RESULTS LAB 3A: DETERMINATION OF WORK FUNCTION AND THRESHOLD FREQUENCY FOR A SPECIFIC METAL USING STOPPING POTENTIAL Set METAL to the selected metal (randomly or specifically selected by student or instructor) Determine the stopping potential for 5 frequencies that include the wide range of the available spectrum Record all observations. Plot a graph of stopping potential (V) versus frequency of incident light (Hz) o Determine the slope of the line of best fit in V∙s and in J∙s (1 eV = 1.6× 10−19 J) o Determine the x-intercept and the y-intercept (include range of uncertainty) Compare your calculated values for work function and threshold frequency with accepted values (using text p. 853 or on-line: Pulse Power or Hyper Physics ) B. REINFORCEMENT ACTIVITY LAB 3B: VERIFICATION OF THE WORK FUNCTION OF A METAL FROM SIMULATION DATA USING CRITICAL FREQUENCY Determine the critical frequency for a metal by changing the wavelength of incident light at 100% intensity while the voltage is zero. Use the formula W = h f0 to determine the experimental value of the work function of the metal Compare the two experimental values with the accepted value (text or on-line source) Evaluate the accuracy and effectiveness of the experiment. C. INDIVIDUAL CONCLUDING ACTIVITY LAB 4: IDENTIFICATION OF AN UNKNOWN METAL BY FROM ITS WORK FUNCTION AND THRESHOLD FREQUENCY Determine the critical frequency for the unknown metal. Use the formula W = h f0 to determine the experimental value of the work function of the metal Compare the experimental value with the accepted value (in text or on-line source) Conclude the identity of the unknown metal with supporting arguments Identify the metal from a list of accepted work function values (in text book or on-line source) Student products expected MiniLab 1: RELATING FREQUENCY AND INTENSITY TO CURRENT MiniLab 2: DETERMINING STOPPING POTENTIAL MiniLab 3A: DETERMINATION OF W ORK FUNCTION AND THRESHOLD FREQUENCY FOR A SPECIFIC METAL Lab 3B: VERIFICATION OF THE W ORK FUNCTION OF A METAL FROM SIMULATION DATA Lab 4: IDENTIFICATION OF AN UNKNOWN METAL BY FROM ITS W ORK FUNCTION AND THRESHOLD FREQUENCY Samples (include teacher notes, assessment information, student work if available) SAMPLE DATA AND GRAPH Logistics (organization, grouping, management issues, access to technology) Computers with Internet and ActiveX o Ensure at least one working computer for every two students o Wireless laptops are preferred (Computer on Wheels) Data projector to demonstrate basic simulation functions with whole group Provide immediate (next class) feedback on expected relationships between variables Assessment information (e.g., rubrics for products and/or process) Graph of Stopping Potential versus Frequency for Sodium Metal created using the Enter Data option from Data Studio line of best fit using the Fit – Linear Fit tool ASSESSMENT RUBRICS MiniLab 3A: Determination of Work Function and Threshold Frequency for a Specific Metal Lab 3B: Verification of the Work Function of a Metal from Simulation Data Lab 4: Identification of an Unknown Metal by from its Work Function and Threshold Frequency QUIZ Possible extensions Adaptations for students requiring additional support Photoelectric Effect – multiple choice quiz Applications of the Photoelectric Effect – Charge Coupling Devices (CCDs) http://web2.uwindsor.ca/courses/physics/high_schools/2005/Photoelectric_effect/applications.html http://www.teachnet.ie/dkeenahan/2005/page31.html http://www.physics.rutgers.edu/ugrad/labs/photoelectric.html APPENDICES SIMULATION EXPERIMENTS MiniLab 1: Relating Frequency and Intensity to Current student worksheet MiniLab 2: Determining Stopping Potential student worksheet Figure 1: Graph of Stopping Potential versus Frequency for Sodium Metal jpg of sample data using Data Studio MiniLab 3A: Determination of Work Function and Threshold Frequency for a Specific Metal marking rubric Lab 3B: Verification of the Work Function of a Metal from Simulation Data marking rubric Lab 4: Identification of an Unknown Metal by from its Work Function and Threshold Frequency marking rubric NOTES Introduction of the Photoelectric Effect Class 1 The Effects of Light Intensity and Frequency Class 2 The Experimental Work of Lenard and the Concept of Stopping Potential Class 2 Einstein, Millikan, Work Function and Threshold Frequency Class 4 QUIZ Photoelectric Effect Multiple Choice Quiz SIMULATION LAB 1: RELATING FREQUENCY AND INTENSITY TO CURRENT Instructions In Internet Explorer, go to phet.colorado.edu then and select OR http://phet.colorado.edu/new/simulations/sims.php?sim=Photoelectric_Effect In the Photoelectric Effect Simulation Set METAL = Sodium and VOLTAGE = 0 V Select wavelengths that include the full range of the available spectrum (200 nm, 300 nm, 400 nm, 500 nm, 600 nm, and 700 nm) Convert wavelength to frequency using f = c / λ . Remember that 200 nm = 2 × 10-9 m and c = 3.0 × 108 m/s. For each wavelength, start with the Intensity at 0% and slowly increase the Intensity to 100%. Record all observations on data sheet (see over) Answer the concluding questions below, play some more with the simulation and record any other findings or conclusions State the relationship between Current and Intensity and predict the shape of the graph of the relationship. Current (A) Conclusions State the relationship between Current and Wavelength and predict the shape of the graph of the relationship. Current (A) Intensity (%) Wavelength (nm) Raw Data Table 1. Effect of changing the intensity of different wavelengths of light on the photoelectrons of sodium metal and the resulting current when the voltage is zero. Sodium Metal Wavelength (nm) Frequency (x 1015 Hz) Colour Minimum Intensity for Photoelectrons (%) 200 300 400 500 600 700 Sample calculation for frequency from wavelength (use 200 nm). Other Findings / Conclusions: Effect of Increasing Intensity Frequency of Speed of Maximum Current Photoelectron Photoelectrons (A) Emission SIMULATION LAB 2: DETERMINING STOPPING POTENTIAL Instructions In Internet Explorer, go to phet.colorado.edu then and select OR http://phet.colorado.edu/new/simulations/sims.php?sim=Photoelectric_Effect In the Photoelectric Effect Simulation Set METAL = Sodium Use the same frequencies (wavelengths) from LAB 1: RELATING FREQUENCY AND INTENSITY TO CURRENT Set the Intensity to 50% For each frequency, slowly change the voltage until the most energetic photoelectrons barely do NOT reach the receiver plate At that “stopping potential” change the intensity. Record all observations in Table 2 (see reverse). At the stopping potential, State the relationship between Stopping Potential and Frequency and predict the shape of the graph of the relationship. State and explain any significant observations Answer the concluding questions below, play some more with the simulation and record any other findings or conclusions Conclusions State the relationship between Stopping Potential and Wavelength and predict the shape of the graph of the relationship. State the effect of Intensity on Stopping Potential Stopping Potential (V) O Wavelength (nm) Raw Data Table 2. Determination of the stopping potential of sodium metal by changing the voltage for different wavelengths of light at 50% intensity. The stopping potential is the voltage at which the current changes to 0. Sodium Metal Wavelength (nm) 200 300 400 500 600 700 Other Findings / Conclusions: Frequency (x 1015 Hz) Colour Stopping Potential (V) GRAPH OF STOPPING POTENTIAL VERSUS FREQUENCY FOR SODIUM METAL Lab 3 : DETERMINATION AND VERIFICATION OF WORK FUNCTION AND THRESHOLD FREQUENCY FOR A SPECIFIC METAL Teacher Name: : _________________________________ Student Name: ______________________________________ CATEGORY 4 3 2 1 Critical Frequency Within 5% of accepted value Within 10% of accepted value Wavelength is accurate (<10% error) but not converted to frequency No critical wavelength found or inaccurate (>10% error) Work Function from Critical Frequency calculated correctly with appropriate units and significant figures minor errors in calculations or significant figures significant calculation errors OR inappropriate units No work function calculated Shows wavelength, Clearly shows wavelength, calculated frequency and calculated frequency and stopping potential with stopping potential with units not shown or not in a units in a logical order logical order Shows wavelength, frequency and stopping potential without sample calculation of frequency Shows wavelength and frequency and stopping potential Data Table Graph of Work Function versus Frequency Complete and accurate with slope, x, and y intercepts clearly labeled Accurate but incomplete with some labels or values missing or unclear Complete and some inaccuracy of values Incomplete labels or many inaccurate values Critical Frequency and Work Function Clearly states both values with appropriate units and significant figures Clearly states both values with inappropriate units or significant figures States only one value appropriately Neither value stated Comparison with Accepted Value Experiment Evaluation Accepted value clearly Accepted value and source identified and percent error Accepted value identified clearly identified and stated but source or but percent error not found percent error found calculation not shown Clearly evaluates the Evaluation the accuracy accuracy and effectiveness and effectiveness without with valid support valid support statements statements TOTAL SCORE: ____ / 28 Either the accuracy or effectiveness was not evaluated or lacked support statements Accepted value not identified No valid evaluation of accuracy nor effectiveness Lab 4 : IDENTIFICATION OF AN UNKNOWN METAL BY FROM ITS WORK FUNCTION AND THRESHOLD FREQUENCY Teacher Name: : Student Name: ________________________________________ ________________________________________ CATEGORY 4 3 2 1 Critical Frequency Within 5% of accepted value Within 10% of accepted value Wavelength is accurate (<10% error) but not converted to frequency No critical wavelength found or inaccurate (>10% error) Work Function from Critical Frequency calculated correctly with appropriate units and significant figures minor errors in calculations or significant figures significant calculation errors OR inappropriate units No work function calculated Shows wavelength, Clearly shows wavelength, calculated frequency and calculated frequency and stopping potential with stopping potential with units not shown or not in a units in a logical order logical order Shows wavelength, frequency and stopping potential without sample calculation of frequency Shows wavelength and frequency and stopping potential Data Table Graph of Work Function versus Frequency Complete and accurate with slope, x, and y intercepts clearly labeled Accurate but incomplete with some labels or values missing or unclear Complete and some inaccuracy of values Incomplete labels or many inaccurate values Critical Frequency and Work Function Clearly states both values with appropriate units and significant figures Clearly states both values with inappropriate units or significant figures States only one value appropriately Neither value stated Comparison with Possible Accepted Values Three most probable metals with accepted values and source clearly identified One possible metal with Two most probable metals accepted values and with accepted values and source clearly identified or source clearly identified two probable metals without source Identity of Unknown Metal Correct identification of Correct identification of unknown with valid reasons unknown without reasons TOTAL SCORE: ____ / 28 Incorrect identification of unknown but valid reasons No possible metals with sources identified Incorrect identification of unknown without valid reasons NOTES UNIT 7 WAVES AND MODERN PHYSICS Chapter 18 Classical Physics Early Quantum Theory the laws, theories, and explanations about mechanics, thermodynamics, electricity and magnetism prior to 1900. − explains most of the observations in our lives − fails to explain the photelectric effect and ultraviolet radiation by a blackbody James Clerk MAXWELL − Electromagnetic Waves Changing electric fields induce changing magnetic fields. Changing magnetic fields induce changing electric fields. Together, the alternating electric and magnetic fields radiate from the source into space. The frequency of the waves is determined by the frequency of either the magnetic field or the electric field that produced the waves. Electromagnetic Radiation Types Energy carried by electromagnetic waves through space All electromagnetic radiation moves at the speed of light, c Radio waves, microwaves, infrared (heat), visible light, ultraviolet light, X-rays, gamma rays, cosmic rays Universal Wave Equation Wavelength (λ) and frequency (f) of the waves are related by the speed of light (c) c=fλ Frequency varies inversely with wavelength. 18.1 The Particle Nature of Light BLACKBODY RADIATION Blackbody An object that absorbs all frequencies of light (= black) An object that emits a complete, continuous spectrum of electromagnetic radiation When an object is heated a little and turns red, it emits infrared (IR) radiation. When the object is heated until it glows white, it emits visible light (ROYGBIV). Kirchhoff Boltzmann All objects absorb the same frequencies of radiation that they emit demonstrated that the power radiated by a blackbody varies with its temperature. used Maxwell’s idea of emission of radiation by vibrating charges on the surface to a blackbody predicted that the energy radiated from a blackbody increased as the frequency increased o true for low energy (infrared and visible) emissions Ultraviolet Catastrophe the huge difference between the predicted energy and the actual energy emitted by higher frequencies of radiation The experimental observations could not be explained by classical physics 840 EARLY QUANTUM THEORY 843 Electrons are believed to move about the nucleus of an atom on discrete energy levels (shells). Electrons can absorb the energy of electromagnetic radiation. If enough energy is absorbed, they jump to a higher energy level (excited state). Eventually, the electrons release energy and return to their starting (ground state) energy level. The energy is released as a packet electromagnetic radiation called a photon. Max Planck German physicist who first realized that the energy released by excited electrons only comes in packets of specific sizes with certain frequencies. E=nhf , where n = energy level integer values only (0, 1, 2, 3,…) h = Planck’s constant 6.626 x 10–34 J / Hz f = frequency (Hertz) INTRODUCTION OF THE PHOTOELECTRIC EFFECT 844 Electrons (photoelectrons) escape from the surface of a metal when light is shone on the metal. The photoelectrons are detected by an ammeter when they strike a metal plate that allows the circuit to be completed. Photoelectric Effect – the emission of electrons from a metal when exposed to electromagnetic radiation. Photoelectric cells use these photoelectrons in a closed circuit. Plants use the photoelectrons in the process of photosynthesis Garage door openers and solar panels operate using photoelectrons Heinrich Hertz discovered the photoelectric effect by accident when sparks jumped between across a separate circuit when a nearby oscillating current Led to development of radio, tv, microwave communication (Wii), and radar THE EFFECTS OF LIGHT INTENSITY AND FREQUENCY 845 Light Intensity only affects the rate that photoelectrons are released (photoelectrons released per second) and does not affect the speed (maximum kinetic energy) of the photoelectrons. Light Frequency determines the amount of energy that can be transferred to each electron (E=hf) which directly affects the kinetic energy (speed) of the photoelectron. THE EXPERIMENTAL WORK OF LENARD AND THE CONCEPT OF STOPPING POTENTIAL 846 Philipp Lenard controlled the potential differences between the incident metal plate and the detector plate. Stopping Potential is the potential difference that will stop all photoelectrons from reaching the detector plate. EINSTEIN, MILLIKAN, WORK FUNCTION AND THRESHOLD FREQUENCY P 847-853 Einstein light behaves as packets of energy called photons the intensity of light affects the number of photons, not the energy of each photon the energy of one photon (E = hf) is transferred to one electron in one event o higher frequency photons (UV radiation) has more energy than IR photons some of the energy transferred is used to do work to overcome the attractive forces in the substance and the remainder was converted to kinetic energy more work is needed for electrons further beneath the surface than those on the surface Work Function – minimum amount of energy required to remove an electron from the surface of a metal (W) Each metal has a distinct work function Maximum Kinetic Energy of a photoelectron is achieved when a surface electron is removed. (EKmax) hf = EKmax + W Millikan experimentally determined the charge of a single electron (qe = 1.6 × 1019C) tried to prove Einstein wrong by precisely measuring the stopping potential for a variety of frequencies of several metals the maximum kinetic energy must be less than or equal to the electrical potential energy EKmax ≤ EP= qe∙Vs plotted maximum kinetic energy versus frequency EKmax = hf – W compare to y = mx + b slope = h = Planck’s constant y-intercept (b) = −W = negative work function x-intercept (a) = f0 = threshold (critical) frequency provided experimental support of Einstein’s proposals of the quantum nature of the photoelectric effect Threshold Frequency (f0) the minimum frequency of the radiation (photon) required for a certain metal to emit a photoelectron. Einstein’s Photon Theory the excess energy of the incoming photon becomes the kinetic energy of the emitted electron. Ek = h f - h f0 where h f is the energy of the incoming photon and h f0 is the minimum energy required to escape (h f0 = W) MEASURING KINETIC ENERGY OF PHOTOELECTRONS IN ELECTRON VOLTS 850 The energy of the photoelectron is measured by balancing its kinetic energy with the repulsive force of a negatively charged anode. The respulsive force depends on the voltage at the anode (stopping potential). EK = – q V0 where EK = the maximum kinetic energy of the electron q = the charge on the electron qe– = –1.60 x 10–19 C V0 = the stopping potential of the anode The kinetic energy of an electron is very small, so electron volts (eV) are used instead of joules. One electron volt is the energy of one electron accelerated against a potential difference of one volt. 1 eV = 1.60 x 10–19 J Practice Problems (p. 561) 3, 4 Quiz: Photoelectric Effect _____ 1. _____ 2. _____ 3. _____ 4. _____ 5. Physics 12 Name: ___________________ What is the term used to refer to the minimum energy required for a photoelectron to escape from a metal plate in a photocell? A. stopping voltage B. Planck’s constant C. threshold wavelength D. work function Threshold frequency is to work function as hertz is to which one of the following? A. coulomb B. joule C. newton D. watt The variable that varies directly with the amount of current produced by photoelectrons A. the intensity of the incident light B. the frequency of the incident light C. the wavelength of the incident light D. the work function of the metal surface The threshold frequency has a value of X. If the frequency of the incident light increases from 2X to 4X, then the resulting current of photoelectrons A. is doubled B. is increased by a factor or 3 C. reduced by half D. remains the same When electromagnetic radiation with a wavelength of 350 nm falls on a metal, the maximum kinetic energy of the ejected electrons is 1.20 eV. What is the work function of the metal? A. 1.3 eV B. 2.4 eV C. 5.4 eV D. 5.7 eV _____ 6. A. 210 nm B. 420 nm C. 530 nm D. 620 nm The graph below shows the relationship between the frequency of radiation incident on a photosensitive surface and the maximum kinetic energy of the emitted photoelectrons. Kinetic Energy (eV) _____ 7. Calculate the wavelength of a photon with 3.2 × 10–19 J of energy. P Frequency (Hz) What does point P represent? _____ 8. A. fundamental frequency B. photoelectron frequency C. photon escape frequency D. threshold frequency The work function of a particular photo-emissive material is 4.0 eV. If photons with 16 eV of energy are incident on the material, what would be the maximum kinetic energy of the ejected photoelectrons? A. 0.25 eV B. 4.0 eV C. 12 eV D. 20. eV