YEAR 11 PHYSICS
advertisement
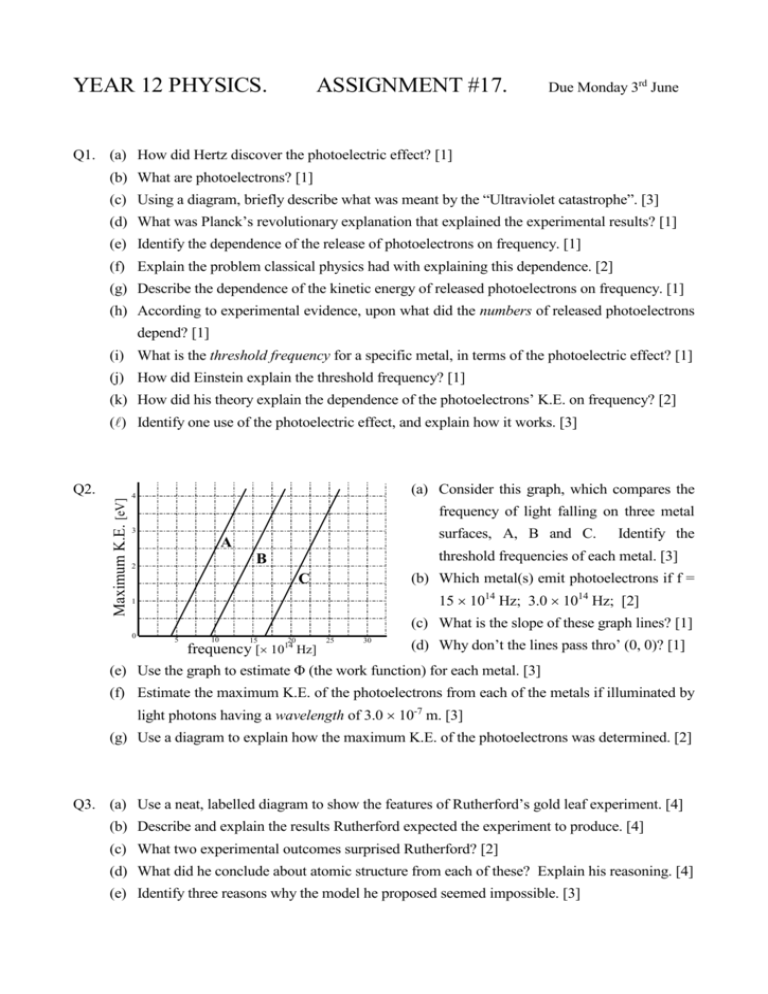
YEAR 12 PHYSICS. ASSIGNMENT #17. Due Monday 3rd June Q1. (a) How did Hertz discover the photoelectric effect? [1] (b) What are photoelectrons? [1] (c) Using a diagram, briefly describe what was meant by the “Ultraviolet catastrophe”. [3] (d) What was Planck’s revolutionary explanation that explained the experimental results? [1] (e) Identify the dependence of the release of photoelectrons on frequency. [1] (f) Explain the problem classical physics had with explaining this dependence. [2] (g) Describe the dependence of the kinetic energy of released photoelectrons on frequency. [1] (h) According to experimental evidence, upon what did the numbers of released photoelectrons depend? [1] (i) What is the threshold frequency for a specific metal, in terms of the photoelectric effect? [1] (j) How did Einstein explain the threshold frequency? [1] (k) How did his theory explain the dependence of the photoelectrons’ K.E. on frequency? [2] () Identify one use of the photoelectric effect, and explain how it works. [3] Maximum K.E. [eV] Q2. (a) Consider this graph, which compares the 4 frequency of light falling on three metal 3 surfaces, A, B and C. A threshold frequencies of each metal. [3] B 2 Identify the C (b) Which metal(s) emit photoelectrons if f = 15 1014 Hz; 3.0 1014 Hz; [2] 1 (c) What is the slope of these graph lines? [1] 0 10 25 15 20 30 (d) Why don’t the lines pass thro’ (0, 0)? [1] frequency [ 1014 Hz] (e) Use the graph to estimate Φ (the work function) for each metal. [3] 5 (f) Estimate the maximum K.E. of the photoelectrons from each of the metals if illuminated by light photons having a wavelength of 3.0 10-7 m. [3] (g) Use a diagram to explain how the maximum K.E. of the photoelectrons was determined. [2] Q3. (a) Use a neat, labelled diagram to show the features of Rutherford’s gold leaf experiment. [4] (b) Describe and explain the results Rutherford expected the experiment to produce. [4] (c) What two experimental outcomes surprised Rutherford? [2] (d) What did he conclude about atomic structure from each of these? Explain his reasoning. [4] (e) Identify three reasons why the model he proposed seemed impossible. [3]











