OLFACTION AS AN ORAL SENSE AND AS A TELECEPTIVE SENSE
advertisement

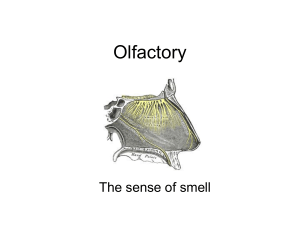
OLFACTION AS AN ORAL SENSE AND AS A TELECEPTIVE SENSE “O God, make me to smell the odours of Paradise, and bless me with its delights, and make me not to smell the smell of the fires of hell.” -Koran, quoted in Moncrieff (1967) The sense of smell has a vividness and evocative quality that are different from any other sense. This may result from the intimate connections of the olfactory system with the paleocortex, or evolutionarily older part of the brain, and with the hypothalamus. It is thus considered to be able to evoke memories which may be processed in the paleocortex (hippocampus and prepyriform cortex), and to have some very primitive functions, in connection with eating and reproduction, that are mediated by the hypothalamic/pituitary system. Most of our subjective judgments of odors have to do with their pleasantness or unpleasantness. This is useful, since things that may be poisonous to eat, such as spoiled meat or rotten eggs, have offensive odors. As mentioned in Chapter 7, estimates of the flavor of food are mostly based on odors, and less on tastes. Olfaction has been the subject of studies in a wide variety of vertebrates and invertebrates (Gesteland et al., 1965; Bang and Wenzel, 1985; Lancet, 1986; Ache, 1987). Much has been written about the role of pheromones which serve as odorous attractants or repellants in insects and higher species (Schneider, 1971). Whether humans consider sweat or other odors attractive is a matter of debate. However, the use of perfumes, which are often floral extracts combined with animal musk, has been practiced for centuries. The olfactory apparatus functions both as an exquisitely sensitive detector of remote smells - for instance in wild animals, to detect the presence of enemies - and also as a method of quality control for foods about to be consumed. The French gastronomist Brillat-Savarin in 1825 wrote that the nose "acts as the first sentinel, crying out, 'Who goes there?'." Anatomy of Olfactory Sensory Cells We do not smell with our noses, but with our olfactory epithelium. As shown in Figure 1, this is where the sensory cells for olfaction in higher vertebrates are located, in the upper and middle conchae of the nasopharynx. The area of this sensitive region varies from a few cm2 in man to 100 cm 2 in dogs (Moulton and Beidler, 1967; Graziadei. 1971). A drawing of the structure of the olfactory epithelium is shown in Figure 2. There are basal cells, supporting (sustentacular) cells, and olfactory sensory (receptor) cells. Olfactory cilia project from the sensory cells into a layer of mucus approximately 35 m thick which covers the sensory epithelium. Molecules of an odorant substance must diffuse across this mucus layer before they can come in contact with the membrane of the olfactory cell, located in the cilia. The main source of the mucus is considered to be the sustentacular cells and the acinar cells of Bowman's glands (Getchell and Getchell, 1987). The olfactory sensory cells are primary sensory neurons. (In the taste system, by contrast, endodermally-derived sensory cells transduce taste stimuli into secretory activity that excites the connected sensory axons.) Electrophysiological and biochemical studies have been done with single isolated olfactory sensory cells and will be described below. There are also endings of the trigeminal system present in the nasal mucosa; these are thought to be the pathway for irritating or "stinging" sensations from the nose. Neural Pathways for Olfactory Signals Which is the first cranial nerve? The axons of the sensory cells are called the fila olfactoria, and pass through the cribriform plate of the ethmoid bone to reach the olfactory bulb. The relay points for the sensory axons of the fila olfactoria are glomeruli in the olfactory bulb, as shown in Figure 5. The glomeruli (a, b, c) contain the dendrites of the large mitral cells (layer C), which are the main afferent pathway for olfaction. Axons of the mitral cells turn in the granular layer (E) and become the olfactory tract. Other important cells are granule cells (I, J), which connect to the mitral cells, and tufted cells (d), whose axons also exit in the olfactory nerve. Some interconnections of the cell types in the olfactory bulb are shown in Figures 6 and 7. The fila olfactoria (FO) synapse with mitral cells (M) and periglomerular cells (PG), in the lowest, or glomerular layer of the bulb. PG-cell axons then synapse on other mitral cells. Granule cells (GR) receive efferent, or centrifugal inputs from the anterior olfactory nucleus (AON) on the same side, and synapse on mitral cells. Mitral cell axons then exit the bulb as the olfactory tract (OT). Recent estimates indicate that about 2000 olfactory sensory cells, coding for a single odorant receptor type, project into a single glomerulus (see for example Ressler et al., 1994). With granule and periglomerular cells, there are many synaptic interactions and possibilities for modifications of the neural olfactory code at the level of the olfactory bulb. The olfactory tract eventually leads to the olfactory bulb on the opposite side, to the prepyriform area and the pyriform lobe, the hippocampus, and, via the amygdaloid complex to the autonomic nuclei of the hypothlamus, where signals may influence the release of hormones involved in reproduction. It is possible to obtain electrical recordings of nerve activity at various parts of the olfactory pathway: Electrodes placed against the surface of the olfactory epithelium record the summed activity of many sensory cells when odorants are applied to the area; this negative-going wave is called the electroölfactogram or EOG. Summed responses of the olfactory nerve may likewise be observed when stimulants are applied to the olfactory epithelium (Adrian, 1950; Gesteland, 1976; Hornung and Mozell, 1981). Action potentials may be recorded extracellularly from single olfactory receptor neurons (Juge et al., 1979a, b). One may also place microelectrodes inside single olfactory receptor cells, and record generator potentials and action potentials when odorants are applied to the preparation (Getchell, 1977a, Suzuki, 1977. Organization of Olfactory System Studied with In-situ Hybridization One may wonder why the neuronal organization of this chemical sense differs from that of taste: there is no analogue of the fila olfactoria in the taste system, but rather a single taste nerve on each side of the mouth. This difference might have to do with the necessity of the olfactory sensory axons to pass through bone before reaching the olfactory bulbs. The complex structure of the olfactory bulb likewise is not seen in the first relay station for taste, the nucleus of the tractus solitarius. With some recent experiments tracing the distribution of the genes for specific receptor molecules in the olfactory system, the function of the bulbs is starting to become clearer: In 1991 Buck and Axel identified proteins in the olfactory epithelium that were coded by a large multigene family, as putative olfactory receptors. In addition, most members of the multigene family were shown to belong to subfamilies ranging in size from one to about 20 genes (Buck, 1993). The individual proteins coded by each subfamily were almost identical in amino acid sequence. The suspicion was, that different subfamilies might recognize different structural classes of odorants. The next development was the finding that different receptor subfamilies were expressed in one of four distinct regions of the olfactory epithelium (Ressler et al., 1993; Vassar et al., 1993). In-situ hybridization methods were used, in which probes are constructed having the same structure as known olfactory receptor molecules, and then applied to sections of the olfactory mucosa or bulb. If the probes find complementary RNA in the sections, they bind by Watson-Crick base pairing. The identified areas of receptor protein in the section are then revealed by a visible or radioactive marker when the probe is washed off. These studies showed that neurons containing the same receptor molecules lay only in one of four different expression zones. This is illustrated in the next slide. Part A shows the turbinates of the rat olfactory epithelium, and Part B indicates the bilaterally symmetrical expression of receptor genes. Several different types of receptors may be produced in a given expression zone, but these types do not occur in other zones. Also, receptors of a given type are not clumped together, but are randomly distributed throughout each single expression zone. The next major finding having to do with connections of the fila olfactoria was somewhat amazing: By looking at hybridization in the olfactory bulb, it was found that specific odorant receptor gene probes hybridize into small and distinct sets of olfactory bulb glomeruli (Ressler et al., 1994; Vassar et al., 1994). This is illustrated in the next slide. Part A shows the hybridization of olfactory marker protein, which binds to all olfactory tissue. B is a magnified view, showing hybridization to specific glomeruli. Then in part E the applied probe is made from olfactory receptor protein M50. Here the probe hybridizes only to certain areas of the olfactory epithelium and only in a few distinct glomeruli. This result, and others with different olfactory receptor proteins, led to the conclusion that while individual sensory neurons expressing a given receptor are distributed throughout the expression zone of the epithelium, they all project to one or a few glomeruli in the olfactory bulb. This indicates there may be a receptor map in the olfactory bulb. By looking at the activity of specific glomeruli, the nervous system is able to identify the stimulus. Chemical Structure and Odor In an analogous manner to the efforts of taste researchers in identifying four (or five or six) basic tastes (Chapter 7), some schemes have been presented wherein classes of odors are related to shapes of odorant molecules (Amoore et al., 1964). One often sees lists of "compounds and their odors" such as the following: Table I. Types of compounds producing various odors. _________________________________________________________________ Odor type Compound _________________________________________________________________ Floral Alcohols e.g. á-phenylethyl alcohol Fruity Esters e.g diethyl adipate Minty Esters e.g. methyl salicylate Camphorous 1,8-cineole Musky Ring ketones, civetone _________________________________________________________________ This is only an attempt to establish classes of basic odors, as for instance both fruity and minty compounds are esters. The more general observation is that higher vertebrates can smell a vast array of different smells, that hardly fall into classes at all (Reed, 1990). Humans can probably distinguish over a thousand different odors (Shepherd, 1990). Amoore and colleagues (1964) tried to find a relation between the shape of an odorant molecule and its odor. This is shown in the next figure. The shape, defined as ratio of major to minor axis in 2 dimensions, was weakly correlated with odor (next figure). Odorant molecules may be polar or nonpolar and may or may not have functional groups attached; this is illustrated in Figure 15. The compound shown in Part VI is a strong musk, whereas the hydrocarbon in Part VII with practically the same molecular shape is odorless. In this case the carbonyl group C=O is necessary for the musk quality to be present. IV and V are both musks, so the functional group is unimportant in this case. Some evidence that suggests the presence of at least some primary odor classes is that of specific anosmias. Some different types of anosmia have been found in humans (Amoore, 1971); for instance, approximately 1% of the European population is insensitive to musks. They evidently are deficient in a gene or have a modified gene for at least one type of olfactory receptor molecule (Lancet, 1986). Davies and Taylor (1959) formed an early theory of olfactory strength, or the amount of an odorant required to reach threshold. They assumed that the threshold depended on the adsorption energy of the odorant molecules going from the air into the lipid-aqueous olfactory cell membranes. From this theory they calculated the olfactory thresholds of several classes of compounds based on known adsorption energies. The sense of smell, working at a distance, is almost as sensitive as is theoretically possible: In some insect chemoreceptors a single molecule of odorant per receptor cell is sufficient to be detected. We can estimate the sensitivity in humans by knowing the concentration of the odorant in the inspired air: For the garlicky compound butyl mercaptan, for instance, the threshold concentration is about 1010 molecules/liter of air. By using estimates of the number of olfactory cells and the volume of a sniff of air, one can calculate the threshold as about eight molecules of mercaptan per sensory cell per sniff. Electrophysiology of Olfactory Sensory Cells Analysis of current flows in olfactory epithelium when electrodes are placed at various depths has suggested that the action potentials arise in an area of the sensory neuron that is relatively remote from the ciliary odorant receptors (Getchell, 1977; Getchell and Getchell, 1987). The mechanism is shown in Figure 17: The transduction current produced by the formation of odorant-receptor complex in the cilia flows centripetally down the sensory cell body. Action potentials are intiated in the cell-body region of the sensory neuron, near the point where the cell tapers in an axon hillock before the axon. More recently, it has been possible to do "patch clamp" experiments with isolated cilia from olfactory sensory cells of toads (Nakamura and Gold, 1987). These reveal the intimate membrane currents produced in the active zones of olfactory cells, when various odorants are applied to them. One interesting result is that the nucleotide cyclic adenosine monophosphate (cAMP) has a direct gating effect on ionic conductances in the membrane (Lancet, 1986; Gold and Nakamura, 1987; Anholt et al., 1989). Cyclic AMP may serve as an intracellular second messenger between olfactory stimulants coming in contact with the sensory cell membrane and the resulting changes in ionic conductances, as outlined in Figure 18. The receptor molecule (R) has a so-called constant (c) and variable (v) region for different odorants. When an odorant occupies the receptor, a GTP-binding protein (G) is activated, which modulates the activity of the adenylate cyclase (C) catalyzing the production of cAMP. This intracellular messenger activates a protein kinase to cause phosphorylation of the ion channel polypeptides, and opening or closing the associated ion channels. This type of cascade has also been suggested for certain taste receptor mechanisms (Chapter 7). It is possible to make suspensions of homogenized olfactory epithelium (Vodyanoy and Vodyanoy, 1987) or detached olfactory cilia (Labarca, et al., 1988) that contain both olfactory receptors and some associated ionic channels. These suspensions may then be added to artificial lipid bilayers membranes, and the behavior of the receptor/channel complexes studied under controlled conditions. In the case of epithelial homogenates, both ATP and GTP must be present in the bathing solutions for the olfactant response to occur. Possible Mechanisms of Odor Discrimination To explain the detection of specific odorants by the olfactory system, current studies have pointed toward the existence of perhaps 1000 different genes coding for specific receptors (Buck, 2000). Each odorant receptor gene is expressed by only ~0.1% of the olfactory sensory neuron population, suggesting that each sensory neuron may express only a single receptor type. This contrasts with color vision, for instance, where only three receptor types are present in the entire population of retinal sensory cells, and taste, where only about four different tastes can be distinguished, suggesting that only a few receptor types exist. The sense of smell may involve many receptors that are able to bind with one or a small number of odorants; since there are so many structurally diverse odorous ligands, it is likely that there would be a large number of different receptor types. Thus, the olfactory sense may detect up to a thousand different odors by using individual receptors. Subfamilies of receptors may detect structurally similar compounds. Receptors of the same type project to only a few glomeruli in the olfactory bulb. Each glomerulus that receives input from a given receptor type is specific for a given epitope on an odorant. Since odorants contain more than one epitope, a particular odorant is recognized by the pattern of glomeruli that it excites. In taste, where each cell expresses many types of receptors, hundreds of different compounds may be detected, but only four of five discriminated. Another difference is that of sensitivity; the threshold for detection of odors is in the micromolar range, while in taste it is in the millimolar range. Psychophysics of Smell Olfactory acuity may be measured by testing subjects' thresholds for detection or recognition of odors. As with other sensory modalities (see Chapter 1), it is possible to measure subjects' perceived sensation magnitudes by asking them to supply a number for each different stimulus strength tested (Cain, 1978). The variation of sensation magnitude with odor intensity (odorant concentration) is best described by a power function = KSn. The value of n varies from odorant to odorant, between 0.2 and about 0.7. Using this measure of sensation magnitude, we can answer two questions about the property of adaptation, or decreasing awareness of smells even though the odorant is still present: (1) What is the time-course of adaptation? and (2) does the sensation decrease to zero with time or to some steady-state level greater than zero? The result is shown in Figure 20. In this case the odorant was n-butyl acetate, which has a fruity odor. The perceived magnitude was the average estimate of a panel of observers. We can see that the adaptation took place in a few minutes, and that the final level was about 30% of the initial. Thus, adaptation alone will not solve the problem of an unpleasant odor. In the United States we generally prefer our environment to be almost odorless. This has resulted from advertising of products to reduce or "kill" odors from foods, animals, etc. The most common method of odor reduction is counteraction, where mixing the malodor with a strong but acceptable smell decreases the perceived odor of both (Cain, 1978). (The method of masking, much less effective, is just to drown out one odor with another.) Thus, pine-scented deodorizers counteract smells from pet stains, etc. simply by making the olfactory apparatus less sensitive to the offending odors. Aging and Olfaction The generally-stated assumption that aging is associated with decreased ability in all the senses may be misleading. For one thing, as mentioned in Chapter 8, the loss of appreciation of food may be due to an impairment of smell rather than taste (Schiffman, 1979; Deems et al., 1991). The age-specific effects on olfaction have recently been studied in a large population (Doty et al., 1984; Doty and Snow, 1988). A survey of the results is shown in Figure 21. The UPSIT value on the ordinate (for University of Pennsylvania Smell Identification Test) is a score that is inversely correlated with recognition thresholds for a group of 40 odorants. Females in the group outperform males at all ages, that is, they can recognize odorants at lower concentrations. Recognition ability is maximal from about 30 - 60 years, then declines steadily. Factors that may contribute to this loss are (1) drying or other alterations of the nasal epithelium, (2) death of receptor cells (Nakashima et al., 1984), and (3) loss of neurons in the olfactory bulb (Smith, 1942). Clearly, the biochemical mechanisms discussed above may also be altered during life. In comparing the chemical senses with others such as touch, then, one is struck with the greater number of steps in the process, both at the level of the sensory ending and of the subsequent neural pathway. Chemical stimuli must first be dissolved in an aqueous medium before encountering the receptors on the cell membrane. The transformed (dissolved) stimuli must then diffuse to the cell membrane area and bind to the receptors, which must be present. Metals or other cofactors may be required. The binding of the ligand to the receptor probably sets off a cascade of reactions involving an intracellular second messenger. If all goes well, ionic channels will be opened and action potentials produced in the sensory pathway. It is not surprising that losses occur in these systems before they do in the skin senses. We shall now consider some of the ways in which our senses are blocked by general and local anesthetics.