Fluidized Bed Experiment: Flow, Pressure Drop, and Fluidization
advertisement
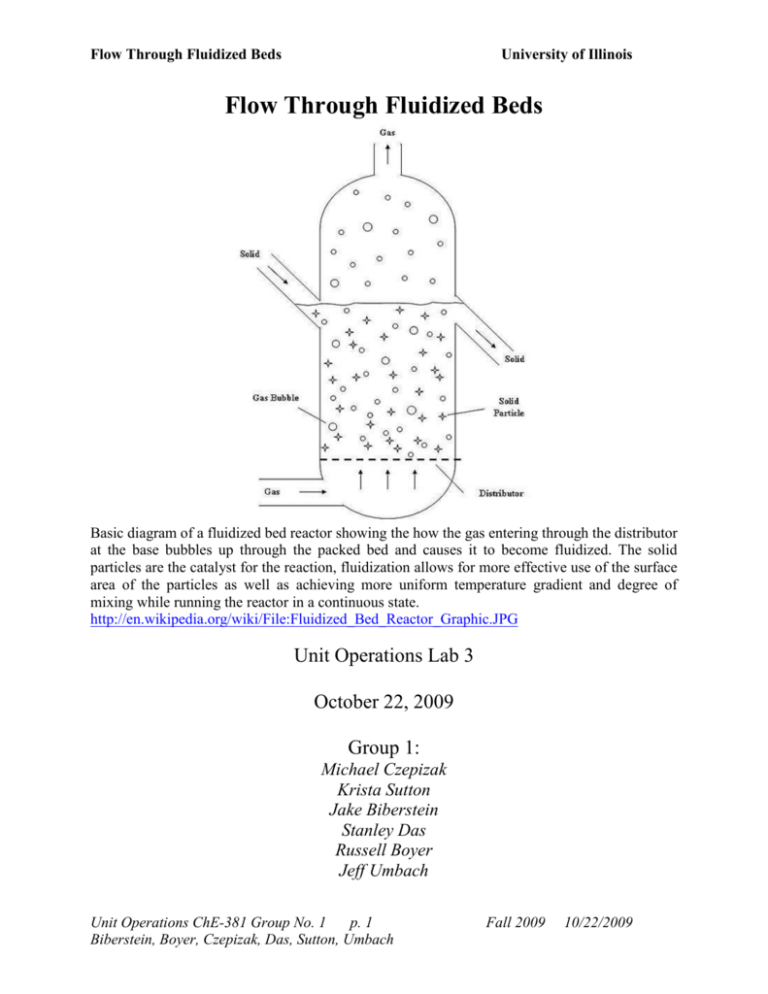
Flow Through Fluidized Beds University of Illinois Flow Through Fluidized Beds Basic diagram of a fluidized bed reactor showing the how the gas entering through the distributor at the base bubbles up through the packed bed and causes it to become fluidized. The solid particles are the catalyst for the reaction, fluidization allows for more effective use of the surface area of the particles as well as achieving more uniform temperature gradient and degree of mixing while running the reactor in a continuous state. http://en.wikipedia.org/wiki/File:Fluidized_Bed_Reactor_Graphic.JPG Unit Operations Lab 3 October 22, 2009 Group 1: Michael Czepizak Krista Sutton Jake Biberstein Stanley Das Russell Boyer Jeff Umbach Unit Operations ChE-381 Group No. 1 p. 1 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 0. Table Of Contents 0. Table Of Contents .................................................................................................. 2 1. WP&C .................................................................................................................... 3 2. Abstract .................................................................................................................. 4 3. Introduction ............................................................................................................ 5 4. Theory .................................................................................................................... 6 5. Apparatus ............................................................................................................. 12 6. Materials and Supplies ......................................................................................... 17 7. Procedure ............................................................................................................. 18 8. Data Tabulation .................................................................................................... 24 9. Results .................................................................................................................. 30 10. Discussion .......................................................................................................... 33 11. Error Analysis .................................................................................................... 36 12. Conclusion ......................................................................................................... 37 13. References .......................................................................................................... 38 14. Appendix I ......................................................................................................... 40 15. Appendix II ........................................................................................................ 40 Unit Operations ChE-381 Group No. 1 p. 2 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 1. WP&C What is the purpose of this experiment? The purpose of Flow Through Fluidized Beds is to measure the effects of particle size, packing amount, packing materials, and heated packing materials on flow through a packed column. We will be working with high-pressure air streams and a heater. Inhalation hazards are unlikely as there is insufficient airflow for packing material to escape from the column. What are the hazards associated with the experiment? Eye and ear damage can result from compressed air streams. Spilled sand or silica can present a slipping hazard. There is a potential burning hazard from the heater in the silica packed column. Be prepared to clean up any broken glass. How will the experiment be conducted in a safe manner? All valves will remain closed until the apparatus has been checked for leaks, buildup of materials, and stoppages. The air inlet valve will be opened and the pressure checked before the valve into the apparatus is opened. What safety controls are in place? The high-pressure air first enters the apparatus through a pressure valve that should be set to approximately 40.0 PSI, not to exceed 100.0 PSI. Each column then has an independent air flowmeter and shutoff valve. Describe safe and unsafe ranges of operations. The range of airflow needed to conduct the experiment is 0.0-1000.0 cc/s of air for the sand packed column and 0.0-13.9 SCFM for the silica packed column. It is not possible to increase the flow beyond these values; if it were possible then doing so would risk damage to sensitive equipment. The pressure range for the apparatus is 0.0-100.0 PSI, which should not be exceeded. I have read the relevant background material for the Unit Operations Laboratory entitled: Flow Through Fluidized Beds and understand the hazards associated with conducting this experiment. I have planned out my experimental work in accordance to standards and acceptable safety practices and will conduct all of my experimental work in a careful and safe manner. I will also be aware of my surroundings, my group members, and other lab students, and will look out for their safety as well. Signatures: _Jeff Umbach_______________________________________ _Russell Boyer_______________________________________ _Michael Czepizak___________________________________ _Krista Sutton_______________________________________ _Jake Biberstein______________________________________ _Stanley Das_________________________________________ Unit Operations ChE-381 Group No. 1 p. 3 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 2. Abstract In this experiment, we measured pressure drop vs. flow rate in two different columns packed with sand or silica at different heights and temperatures. Theoretically, the superficial velocity Vs should equal the air velocity at minimum fluidization Vf. For the sand trials, we found that an average Vs of 0.045 +/- 0.011 meters/second for the large grain sand. The small grain sand had an average Vs of 0.034 +/- 0.0083 meters/sec. For silica, the Vs values were 0.440 + /- 0.109 meters/second and 0.321 +/- 0.080 meters/second. The Vf values for the small grain sand were very close to the theoretical values with an average of 0.035 +/- 0.0082 meters/sec. The Vf values for the large grain sand did not fit the theoretical values. The Vf values for the large grain sand averaged out to 0.110 +/- 0.027 meters/second, which is more than twice the Vs value. The Vf values for silica were 0.50 +/- 0.124 meters/second and 0.48 +/- 0.119 meters/second for unheated and heated silica respectively. These values are close to the Vs values, but they are not as accurate as the values obtained for the smaller grain size of sand. In general, the pressure drop in the column was greater when more material was added to the column bed. Also, the heated silica fluidized much faster than the room temperature, which would be expected due to lower densities of air particles at higher temperatures. Unit Operations ChE-381 Group No. 1 p. 4 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 3. Introduction Fluidized bed reactors are a relatively new tool in the chemical engineering field. Fritz Winkler developed the first fluidized bed gas generator in Germany in the 1920s. One of the first United States fluidized bed reactors used was the Catalytic Cracking Unit, created in Baton Rouge, LA in 1942 by the Standard Oil Company (Exxon Mobil). A fluidized bed is a packed bed through which fluid flows at such a high velocity that the particles in the bed are loosened such that the bed behaves as though it is a liquid. Fluidized beds provide a large surface area for contact between solids and a liquid or a gas that is conducive for heat and mass transfer. In this environment, nearly uniform temperatures can be maintained in the reactor even with highly exothermic reactions. This is important because a temperature gradient can form in a poorly mixed bed, leading to equipment failure, product degradation, and decreased efficacy of the reaction. A fluidized bed also provides uniform mixing, which is important for product quality and efficiency. Fluidized bed reactors are often a continuous process, meaning they are also very efficient compared to batch processes. However, there are some disadvantages to fluidized beds. One disadvantage is that the cost of a fluidized bed reactor is usually high because the vessels are typically larger than batch or other processes. Another disadvantage is that sometimes particles may become entrained, or blown along with the flow, which can be costly and problematic to repair. There is also an extra power input that is required for the pump to moderate the pressure drop. Finally, the fluid-like behavior of these fine particles may eventually cause erosion issues. Fluidized beds can be stimulated by either gas or liquid flows. In either case, the process of fluidization is a competition between the force of gravity pointing downwards and the upward Unit Operations ChE-381 Group No. 1 p. 5 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois pointing drag force caused by friction between the flowing fluid and the individual particles that make up the fluidized bed. As the flow rate of the working fluid is increased, it flows faster across the individual particles, increasing the magnitude of the drag force. Eventually, at a certain velocity (called the minimum fluidization velocity, Vf), the drag and gravitational forces will be in balance, and the bed will begin to fluidize and bubble. As the velocity is further increased, the drag force becomes more and more dominant over gravity, and the bed bubbles more furiously. The individual particles are not carried away with the flow, because their settling velocities are far larger than the minimum fluidization velocity, perhaps 50-75 times larger. In this experiment, minimum fluidization velocity will be found for several different types of fluidized beds. The effects of pressure drop, bed height, bed type, grain size, and temperature will be investigated. 4. Theory In packed beds the Ergun equation is a very important relation. The Ergun equation relates the friction factor to numerical constants and the Reynolds Number (Re). f p 150 Re 1.75 (1) Where: fp = friction factor of bed (dimensionless) Re = Reynolds number (dimensionless) The Reynolds number for this scenario is defined as follows: Re D pVs f (1 ) Unit Operations ChE-381 Group No. 1 p. 6 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach (2) Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois Where: Re = Reynolds number (dimensionless) Dp = Equivalent spherical diameter of the particle (m) Vs = Superficial velocity (m/s) = Density of the fluid (kg/m3) ε = Void fraction of the bed (dimensionless) μ = Dynamic viscosity of the fluid (Pa-s) The equivalent spherical diameter of the particle is defined by: Vp D p 6 * SAp (3) Where: Dp = Equivalent spherical diameter of the particle (m) Vp = Volume of the particle (m3) SAp = Surface area of the particle (m2) Notice that the Reynolds number depends on the void fraction (ε). The void fraction is the ratio of the void volume to the total volume of the bed. Common values for the void fraction range between 0.4-0.45. If the flow is very viscous and Re ≤ 1 then the Ergun equation (1) can be approximated to the Kozeny-Carman Equation: f p 150 Re Unit Operations ChE-381 Group No. 1 p. 7 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach (4) Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois Where: fp = friction factor of bed (dimensionless) Re = Reynolds number (dimensionless) The form of the Ergun equation given in equation (1), while true, is not in terms of variables that are easily measured. We must derive a relation between the fundamental force balance of fluidization and the minimum fluidization velocity. Using the force balance, and starting with the drag force on the bed from the fluid: F pA (5) Where: F p A = Drag force exerted by fluid on the bed (N) = Change in pressure across the bed (N/m2) = Cross-sectional area of the bed (m2) This gives us the upward pointing drag force acting on the bed. In fluidization, this force is balanced with the downward force of gravity, a volumetric force. We must first get the volume of the particles in the bed: V p (1 ) AL (6) Where: Vp = Volume of the bed (m3) ε = Void fraction of the bed (dimensionless) A = Cross-sectional area of the bed (m2) Unit Operations ChE-381 Group No. 1 p. 8 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds L = University of Illinois Height of bed (m) Knowing that the force of gravity is volume multiplied by density and the gravitational constant, we can turn equation 6 into: F ( p f )(1 ) ALg (7) Where: F = Gravitational force on the bed (N) = Density of the bed (kg/m3) = Density of the fluid (kg/m3) ε = Void fraction of the bed (dimensionless) A = Cross-sectional area of the bed (m2) L = Height of bed (m) g = gravitational constant (9.8 m/s2) Knowing that, at minimum fluidization velocity, the drag and gravitational forces are equal, we can set equations 5 and 7 equal to one another, and rearrange for the pressure drop, which is something the apparatus can measure: p ( p f )(1 ) Lg (8) Where: p = Change in pressure across the bed (N/m2) = Density of the bed (kg/m3) Unit Operations ChE-381 Group No. 1 p. 9 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois = Density of the fluid (kg/m3) ε = Void fraction of the bed (dimensionless) L = Height of bed (m) g = gravitational constant (9.8 m/s2) At minimum fluidization velocity, viscous forces dominate, so the Kozeny-Carman equation (4) can be used to get a useful approximation for the minimum fluidization velocity. However, we need an expression relating the friction factor to the pressure drop: fp p D p L Vs2 ( 1 ) 3 (9) Where: fp = friction factor of bed (dimensionless) = Change in pressure across the bed (N/m2) L = Height of bed (m) Dp = Equivalent spherical diameter of the particle (m) ρ = Density of the fluid (kg/m3) Vs = Superficial velocity (m/s) ε = Void fraction of the bed (dimensionless) p Substituting equation 8 into equation 9 (for the delta-p term), then using equation 4 along with equation 2 yields, after rearrangement: Vf ( p f ) gD2p 150 ( 3 1 ) Unit Operations ChE-381 Group No. 1 p. 10 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach (10) Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois Where: Vf = Minimum fluidization velocity (m/s) = Density of the particles (kg/m3) = Density of the fluid (kg/m3) g = gravitational constant (9.8 m/s2) Dp = Equivalent spherical diameter of the particle (m) μ = Dynamic viscosity of the fluid (Pa-s) ε = Void fraction of the bed (dimensionless) Our goal of determining a way to find the minimum fluidization velocity in terms of variables that can be measured is complete. If we wish not to entrain our particles into the flowing fluid stream, it’s useful to know the settling velocity of the particles involved. Vsettling ( p f ) gD2p 18 (11) Where: Vsettling = Settling velocity of the bed (m/s) = Density of the bed (kg/m3) = Density of the fluid (kg/m3) g = gravitational constant (9.8 m/s2) Dp = Equivalent spherical diameter of the particle (m) μ = Dynamic viscosity of the fluid (Pa-s) Unit Operations ChE-381 Group No. 1 p. 11 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois It’s also useful to know the maximum value the velocity can be increased to without entraining particles. Expressed as a multiple of the minimum fluidization velocity: Vsettling Vf 25 (1 ) 3 3 (12) Where: Vsettlin g = Vf The ratio of settling velocity to the minimum fluid fluidization velocity Vsetting = Settling velocity (m/s) Vf = Minimum fluidization velocity (m/s) ε = Void fraction of the bed (dimensionless) In this experiment, we are indirectly finding the minimum fluidization velocity, V f, by increasing the gas flow rate until the pressure drop no longer increases, then decreasing the flow rate until the pressure drop returns. From this information, as well as recording the pressure drop, grain size, and void fraction of the bed used, we can analytically calculate the minimum fluidization velocity necessary to fluidize the bed, and can draw out any correlations between this velocity, grain size of the bed used, and temperature of the bed. 5. Apparatus The Fluidized Bed Apparatus consists of two columns each with a wood packed bottom (6) and a funnel (9). One is packed with sand (5) and the other with silica pellets (14). Each Column has an air rotameter (3,18), which monitors the flow rate of the air entering the column. The air enters though the bottom of the columns and flows upward through the wood beads, Unit Operations ChE-381 Group No. 1 p. 12 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois which distribute the airflow evenly throughout the width of the column. The air then flows through the packing where the air manometer (8) measures the pressure drop of the air stream. The sand column is used for experiments in which the variable if interest is the size of the packing material or the amount of packing material. The sand is sieved using different grates to separate the particles by size. A meter stick is used to measure the height of the packed sand in the column when varying the amount of packing material. The silica column is used in addition to the sand column to test different packing materials. It is also used in varying the temperature of the packing media. The peanut heater (20) is connected to the silica packed portion of the column (16) and is used to heat the packed silica to different temperatures. The thermocouples (16) and the thermometer (15) are used to make sure the entire packed portion is uniform in temperature. Unit Operations ChE-381 Group No. 1 p. 13 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois Figure 1. The figure above shows a flow diagram of the Flow through Packed Beds and Fluidized Beds Apparatus. The general streams pictured in black represent the air streams. The yellow stream at the top left is the sieved sand of specific diameter. The grey stream at the top right is the silica pellets. Unit Operations ChE-381 Group No. 1 p. 14 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 9 Funnel 9 Funnel 3 Air Rotameter 8 Air Mamometer 4 Sand Packed Column 18 Air Rotameter 14 Silica Packed Portion 2 Pressure Gauge 5 Sand Packed Portion 6 Wood Packed Portion 17 Air inlet Valve 6 Wood Packed Portion 7 Flask 1 Air Inlet Valve Figure 2. The picture above shows the Flow through Fluidized Bed apparatus. Note: there have been changes made to the apparatus since captured. The top rim on the silica column was loose and needed to be repaired. http://images.google.com/imgres?imgurl=http://www.uic.edu/depts/chme/UnitOps/Humidification.jpg Unit Operations ChE-381 Group No. 1 p. 15 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds No. 1 2 Equipment Air Inlet Valve Pressure Gauge University of Illinois Table 1. Apparatus Manufacturer Description Crane Co. CAT No. 7 Allows air into the sand packed column N/A 3 Air Rotameter Gilmont D_6626 4 Sand Packed Column N/A 5 Sand Portion of Column N/A 6 Bead Packed Base of Column N/A 7 Flask N/A 8 Air Manometer Meriam Instrument, Cleavland OH Mo: RC4615 9 Funnel N/A 10 Excess Air Stream N/A 11 Sieve Dual Manufacturing Co. Chicago IL (63-4760 Microns) 12 Sand Entrance N/A 13 Silica Entrance N/A 14 Silica Portion of Column N/A 15 Digital Thermometer Fluke. Omega Engineering Inc: Stanford CT. Mo:2166A 16 Thermocouples N/A 17 Air Inlet Valve 18 Air Rotameter Crane Co. CAT No. 7 F&P Co. Precision Bore Flowrator Tube No: FP Monitors the pressure of the incoming air Monitors the incoming air flow rate for the sand packed column Uses sand as a packing material. The amount of sand and the diameter of the sand particles can be varied in this column This is the portion where the packing material settles Allows incoming air into the column and disperses the air stream using packing to create a relatively consistent stream though out the diameter of the sand portion Connected to the packing material portions of the columns this filters out excess packing material from the air Measures the pressure loss of the air stream The top of the column. Facilitates adding packing material to the column and prevents spilling caused by high pressure air streams The excess air from the column comes out the top into the surroundings Used to filter sand using different grates so that a certain range in diameters can be obtained The sand will get sieved then funneled into the column from the top the silica packing with be funneled through the top of the column this is where the silica will reside while the experiment is running Measures the temperature through the packed portions of the column to make sure temperature remains the same Measure the temperature in the silica packed column. Connected to the digital thermometer. Allows air into the silica packed column Monitors the incoming air flow rate for the silica packed column Unit Operations ChE-381 Group No. 1 p. 16 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 3/4-21-G-10/83 Pressure Gauge N/A Monitors the pressure of the incoming air 20 Peanut Heater Superior Electric Co: Bristol, Conn. Powerstat Variable transformer 3PN116B 120 V, 10 Amp Used to heat the air and packing material in the silica column in order to measure the effects of temperature on airflow rate 21 Small Air Valve Crane Co. CAT No. 7 22 Silica Packed Column N/A 19 23 24 Silica Column Valve Sand Column Valve Located after the Pressure Gauge to protect the air rotameter from harmful air flows. Uses silica pellets as a packing material. The temperature of the packing material can be varied in this column N/A Allows are to flow into the packed column. N/A Allows are to flow into the packed column. 6. Materials and Supplies Table 2. Materials and Supplies Material Name Manufacturer Description Safety/Comments Compressed Air UIC Compressors Provides a constant stream of air which flows through the packed column The pressure should be regulated as high pressure streams are hazardous both to participants and equipment Silica Pellets Sand Sieves N/A Fisher Chemical Lot No. 080318 Dual Manufacturing Co: 63 Microns-4760 Microns Electricity ComEd Meter Stick N/A Packing material used to measure affects of temperature of stream on air flow through packed beds Packing material used to measure affects of particle size on air flow though packed beds Avoid spilling to prevent a tripping hazard Avoid spilling to prevent a tripping hazard These cylinders with different size grates sift sand according to range in diameter Do not leave out as excess sand may spill creating a tripping hazard The heater for the air stream in the silica bed needs electricity to heat the air stream Measures the amount of Circuits should be avoided if there is a problem seek an electrician Should be put back properly Unit Operations ChE-381 Group No. 1 p. 17 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds Broom N/A Vacuum Dayton: Mo. 4YE74 University of Illinois material in the sand packed column Used to sweep up any spilled sand or silica The vacuum is used to clean the columns in order to vary the size or amount of the sand. as is could fall and cause injury Prevents tripping hazards Spilling may occur while vacuuming 7. Procedure Things to Know Before You Start 1. The entire lab shares the air supply. If other experiments requiring the air supply are being run at the same time as yours then you will have issues with maintaining steady air pressure. Keep an eye on the flowmeter at all times. You may need to coordinate with other lab groups in order to secure the amount of airflow that you need. 2. There is insufficient pressure to operate both columns simultaneously, so perform the experiment on only one column at a time. a. As it can take a long time for the heater (20) in the right column (22) to achieve steady state, we recommend first performing the silica experiment at room temperature and then performing the sand experiments in the left column (4) while the right column (22) heats up. Make sure that someone keeps an eye on the temperature of the right column. Start Up 1. Check all connections for air and electrical supply. Unit Operations ChE-381 Group No. 1 p. 18 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 2. Check that all apparatus and materials in the cabinet are accounted for and that nothing is broken. Make sure that the temperature probes are connected to the digital thermometer (15). Make sure that you have a Shop-Vac (vacuum cleaner) for cleanup later. 3. Clean out the left (4) and right (22) columns if they have not been already. 4. After making sure that all other airflow valves (1, 17, 21, 23, 24) on the apparatus are closed, turn on the main air supply valve. Packing Material Preparation 1. Using the sieve trays (11), prepare separate samples of sand and silica that are of the approximate diameter required for the experiment. The sand will be placed in the left column (4) while the silica will be placed in the right column (22). 2. You will prepare two samples of sand that are of two different particle diameters and one sample of silica. One sand sample should be between 63-297 microns and the other should be between 297-595 microns. The silica sample should have a grain size ranging from 500-841 microns. Record the diameter range for each sample in your data. 3. To perform the sifting, choose a two sieve trays (in order from smallest to largest with the largest on top) and one catch tray. These trays stack atop each other with the bottom sieve being the of the lowest grain diameter that you need, the top sieve is of the highest grain diameter that you need. You will use the sifted material that is captured in the bottom sieve tray (not the catch tray) for your sample. Repeat this step for each sample using the appropriate sieve trays. Unit Operations ChE-381 Group No. 1 p. 19 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois a. Note: You will at least 2.5 to 3 times as much silica than sand, as you will need to fill the entire heater assembly (14) in the right column (22) before you will be able to see enough of the silica bed to visually measure its height. b. Note: Save at least 5ml of each sample (using a beaker or graduated cylinder to measure) for the determination of the void fraction. Determining Void Fraction 1. For each sample, fill a graduated cylinder with up to 5ml of the sample material. 2. Measure separately 5ml of water. Add the water to the graduated cylinder and allow a few minutes for the water to completely soak into the material. 3. Measure the total volume in the graduated cylinder. 4. Use the dry volume of the packing material, the volume of the water that was added to the packing material, and the total volume measured after the material has soaked thoroughly to calculate the void fraction of the packing material. 5. Repeat for all samples. Experimental Procedure for Sand 1. Load one sample of the sand packing material into the left column (4) using the funnel at the top to keep from spilling. a. The height of the packing material in the column should be between 6 to 10 cm. Accurately measure this height from the base of the bed, just above the gas distributor (6) at the bottom of the column. Unit Operations ChE-381 Group No. 1 p. 20 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois b. While pouring the packing material into the column, tap the side of the column to make sure that the material does not stick to the side. This will also allow for the packing material to settle more evenly at the base of the column. 2. Open the airflow valve (23) at the base of the left column (4) and make sure that the airflow valve (24) at the base of the right column (22) is turned off. 3. Using the flowmeter (3) on the left side of the apparatus, slowly open the larger valve (1) below the flowmeter and then gently turn the smaller valve (21) at the bottom of the flow meter to increase the flow in increments of 5%. (Note: On this meter, each increment is a percentage of the max flow rate of 2.2 L/s.) c. Note: The flow meter adjustments can be tricky, if you go past the change you were attempting for do not go back down or you will skew your results. 4. For each change in airflow rate record: the air flow rate, the pressure drop on the left side of the manometer (8), the height of the packed bed (5) using the meter Stick, and your visual observations. 5. Keep increasing the airflow rate until you reach the maximum for this bed of material. You will know that this has been achieved when the bed is completely fluidized and further increases in airflow rate do not cause further significant drops in pressure on the manometer (8). Record the maximum flow rate, bed height, and pressure drop. 6. Decrease the airflow rate in increments of 5 on the flowmeter (3). Record the airflow rate, bed height, and pressure drop for each change in airflow rate. 7. Once the airflow is turned off, add more of the sand sample that you were testing to the left column (4) and increase the height of the bed by 3 to 4 cm. Repeat steps 2 through 6. Unit Operations ChE-381 Group No. 1 p. 21 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 8. Once finished with this sample, remove the funnel and use the Shop-Vac to remove all of the sand from the left column (4). Then perform steps 1 through 7 using the second sand sample. Experimental Procedure for Silica 1. Load one sample of the silica packing material into the right column (22) using the funnel at the top to keep from spilling. d. The height of the packing material in the column should be between 6-10 cm above the top of the heater unit at the base of the column, at least 2-3 cm above the highest thermal sensor. Accurately measure this height from the base of the bed, just above the gas distributor (6) at the bottom of the column. This includes the heater area (14). e. While pouring the packing material into the column, tap the side of the column to make sure that the material does not stick to the side. This will also allow for the packing material to settle more evenly at the base of the column. 2. Open the airflow valve (24) at the base of the right column (22) and make sure that the airflow valve (4) at the base of the left column (23) is turned off. Make sure that the electrical heater (20) is turned off. 3. Use the digital thermometer (15) to record the temperatures of the right column (22). There are temperature probes (16) mounted at multiple positions in the silica bed. 4. Using the flowmeter (18) on the right side of the apparatus, slowly open the valve (17) below the flowmeter 5%. (Note: On this meter, each increment is a percentage of the max flow rate of 139.0 SCFM.) Unit Operations ChE-381 Group No. 1 p. 22 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois f. Note: The flow meter adjustments can be tricky, if you go past the change you were attempting for do not go back down or you will skew your results. 5. For each change in airflow rate record: the air flow rate, the pressure drop on the right side of the manometer (8), the height of the packed bed using the meter Stick, the temperatures of the column, and your visual observations. 6. Keep increasing the airflow rate until you reach the maximum for this bed of material. You will know that this has been achieved when the bed is completely fluidized and further increases in airflow rate do not cause further significant drops in pressure on the manometer (8). Record the maximum flow rate, the temperatures of the column, bed height, and pressure drop. 7. Decrease the airflow rate in increments of 5 on the flowmeter (18). Record the airflow rate, the temperatures of the column, bed height, and pressure drop for each change in airflow rate. 8. Once the air flow is turned off, turn on the heater (20) and allow the silica bed to reach a steady state at a temperature about 30 to 40 degrees higher than in the previous test. Use the digital thermometer (15) to monitor the temperatures in the silica bed. (Note: Higher temperatures than this are more difficult to maintain at a steady state.) Repeat steps 4 through 8. 9. Optional: If you have time to allow the column to cool back down to room temperature, add more of the silica sample that you were testing to the right column (22) and increase the height of the bed by 3 to 4 cm. Repeat steps 2 through 8. Unit Operations ChE-381 Group No. 1 p. 23 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 10. Optional: Once finished with this sample, remove the funnel and use the Shop-Vac to remove all of the silica from the right column (22). Then perform steps 1 through 9 using the second silica sample. 11. You will not likely have time for steps 9 and 10. Shut Down and Clean Up 1. Turn off the air supply. Make sure that the heater (20) is turned off. Turn off the digital thermometer (15). 2. Remove the funnel from the top of each column. Use the Shop-Vac to vacuum out all packing material from inside each column. Put the funnel back in place when done. 3. Clean each sieve tray. Do not use water as the trays may corrode! 4. Place all materials in the cabinet below the apparatus and close the door. 8. Data Tabulation The data presented in Table 3 corresponds to a large sand particle diameter in the range of 297595 microns and an initial bed height of 7.31 cm. Flow Rate (% of 2.2 L/s, +/- 1%) 0 3.8 7 14.9 19 24.5 34.1 Table 3. Pressure Drop across Large Sand Particles Trial 1 Pressure Drop Sand Height Observations (+/- 0.01 in H2O) (+/- 0.25 cm) -0.59 47.13 Stable, surface not bubbling at all -0.46 47.15 Stable, surface not bubbling at all -0.31 47.15 Stable, surface not bubbling at all -0.09 47.15 Stable, surface not bubbling at all 0.01 47.15 Stable, surface not bubbling at all 0.19 47.16 Stable, surface not bubbling at all 0.12 47.36 Slight bubbling Unit Operations ChE-381 Group No. 1 p. 24 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds 45 52.7 60.2 69.7 76.9 83.3 91.8 96.9 89.2 82.6 66.8 58.1 49.7 40 32.5 28.1 25.5 12 2.9 0 0.15 0.18 0.22 0.28 0.29 0.31 0.31 0.31 0.31 0.31 0.27 0.23 0.2 0.11 0 -0.02 -0.02 -0.08 -0.43 -0.43 University of Illinois 47.44 47.82 48.27 48.47 48.57 48.68 48.92 49.05 48.87 48.76 48.18 48.12 48.15 47.87 47.8 47.71 47.55 47.4 47.4 47.23 Near even mixing Slightly higher than even mixing Slightly higher than even mixing Low turbulent mixing Turbulent mixing High turbulent mixing High turbulent mixing Very high turbulent mixing High turbulent mixing High turbulent mixing Just above even mixing Even mixing Just less than even mixing Just above minimum fluidization Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all The data presented in Table 4 corresponds to a large sand particle diameter in the range of 297595 microns and an initial bed height of 10.19 cm. Table 4. Pressure Drop across Large Sand Particles Trial 2 Air Flow Rate (% Pressure Drop Sand Height Observations of 2.2 L/s, +/- 1%) (+/- 0.01 in H2O) (+/- 0.25 cm) 0 -0.62 50.01 Stable, surface not bubbling at all 5 -0.25 50.05 Stable, surface not bubbling at all 10.8 0.06 50.06 Stable, surface not bubbling at all 24.6 0.9 50.17 Stable, surface not bubbling at all 34 1.01 50.2 Slight bubbling 44.9 1.01 50.65 Slightly more bubbling 52.2 1.01 50.87 Almost even mixing 63 1.06 51.21 Slightly turbulent mixing 73 1.11 51.68 Turbulent mixing 85.3 1.2 52.15 High turbulent mixing 93.1 1.2 52.38 Violent mixing 99 1.21 52.48 Violent mixing 85.11 1.2 52.25 Violent mixing 78.8 1.2 52.11 High turbulent mixing 73.8 1.19 51.98 Turbulent mixing Unit Operations ChE-381 Group No. 1 p. 25 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds 66 51.2 44 34 5.9 2.1 0 1.19 1.01 0.8 0.63 -0.31 -0.58 -0.59 University of Illinois 51.83 51.8 51.31 51.18 51.05 50.85 50.45 Just above even mixing Very slight bubbling Very slight bubbling Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all The data in Table 5 corresponds to a silica particle diameter in the range of 63 to 297 microns, a range in temperature from 69F to 78F, and an initial bed height of 6.63 cm. Table 5. Pressure Drop across Small Sand Particles Trial 1 Air Flow Rate (% Pressure Drop Sand Height Observations of 2.2 L/s, +/- 1%) (+/- 0.01 in H2O) (+/- 0.25 cm) 0 -0.6 46.45 Stable, surface not bubbling at all 4.3 -0.45 46.46 Stable, surface not bubbling at all 11.2 -0.28 46.46 Stable, surface not bubbling at all 14.2 -0.2 46.48 Stable, surface not bubbling at all 19.4 -0.08 46.48 Stable, surface not bubbling at all 32 0.01 47.21 Even mixing 36.9 0.01 47.28 Slightly more than even mixing 42.3 0.01 47.32 Slightly more than even mixing 50.3 0.02 47.42 Fairly turbulent mixing 61.8 0.08 47.49 High turbulent mixing 68.8 0.1 47.95 Violent mixing 65.9 0.09 47.74 High turbulent mixing 57 0.08 47.5 Fairly turbulent mixing 50 0.04 47.41 Slightly more than even mixing 38.2 0.01 47.35 Even mixing 27 -0.01 47.2 Slight bubbling 21.8 -0.1 46.95 Very little bubbling 6.7 -0.4 46.6 Stable, surface not bubbling at all 0 -0.58 46.46 Stable, surface not bubbling at all The data in Table 6 corresponds to a silica particle diameter in the range of 500 to 841 microns, a range in temperature from 69F to 78F, and an initial bed height of 19.15 cm. Air Flow Rate (% of 2.2 L/s, +/- 1%) Table 6. Pressure Drop across Small Sand Particles Trial 2 Pressure Drop Sand Height Observations (+/- 0.01 in H2O) (+/- 0.25 cm) Unit Operations ChE-381 Group No. 1 p. 26 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds 0 6 14 20 28.1 35 43.2 53.4 65 75.6 70.9 66.1 57.8 51.7 47.2 40.9 33.2 23.3 11 7.6 3.9 0 -0.59 0.31 1 1.51 1.22 1.31 1.33 1.4 1.51 1.51 1.52 1.51 1.49 1.49 1.46 1.41 1.21 0.72 0.5 0.2 -0.07 -0.6 University of Illinois 50.92 50.98 51.08 51.22 52.05 52.45 52.53 53.1 53.56 53.98 53.52 53.42 53.38 53.19 53.05 52.85 52.72 52.6 51.83 51.3 50.97 50.92 Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all Slight bubbling Slightly more bubbling Almost even mixing Just above even mixing Turbulent mixing Very turbulent mixing Very turbulent mixing Very turbulent mixing Turbulent mixing Turbulent mixing Slightly turbulent mixing Above even mixing Even mixing Slight bubbling Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all Stable, surface not bubbling at all The data in Table 7 corresponds to a silica particle diameter in the range of 500 to 841 microns, a range in temperature from 69F to 78F, and an initial bed height of 19.15 cm. Table 7. Pressure Drop across Silica in the Temperature Range 69F to 78F Air Flow Rate (% Pressure Drop Silica Height (+/of 139 CFM, +/Observations (+/- 0.01 in H2O) 0.25 cm) 1%) 0 -0.62 59.13 Stable, surface not bubbling at all 7.2 1.63 59.14 Stable, surface not bubbling at all 9 1.8 59.14 Stable, surface not bubbling at all 10 1.9 59.14 Stable, surface not bubbling at all Very slight movement on silica 12.1 2.32 59.14 surface More noticeable movement on 14 3.05 59.14 surface More noticeable movement on 15.1 3.3 59.14 surface More noticeable movement on 16.4 3.7 59.14 surface Unit Operations ChE-381 Group No. 1 p. 27 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 19.2 4.04 59.17 21.2 23 27 31.8 35.9 31.5 29.8 26.9 25.2 24 21.8 21 19.1 17.9 14.6 12 9.3 6.8 0 4.04 4.06 4.1 4.1 4.18 4.14 4.11 4.1 4.09 4.08 4.02 3.95 3.98 3.91 3.4 2.87 2.2 1.1 -0.6 59.43 60.01 60.42 61.23 62.65 61.45 60.94 60.45 60.31 60.14 59.87 59.72 59.19 59.13 58.6 58.6 58.57 58.57 58.57 Near even mixing; more bubbling than before Slow bubbling but large bubbles Even mixing Turbulent mixing Violent mixing Extremely violent mixing Violent mixing Highly turbulent mixing Turbulent mixing Slight turbulent mixing Just above even mixing Even mixing Just above minimum fluidization Minimum fluidization Slight movement Slight movement Slight movement Very minimal bubbling Stable, surface not bubbling at all Stable, surface not bubbling at all The data presented in Table 8, corresponds to a silica particle diameter in the range of 500-841 microns and an initial bed height of 18.34 cm. Table 8. Pressure Drop across Silica in the Temperature Range 88F-108F Air Flow Rate (% Silica Pressure Drop of 139 CFM, +/Height (+/Observations (+/- 0.01 in H2O) 1%) 0.25 cm) Stable, surface not bubbling at 0 -0.6 58.32 all Stable, surface not bubbling at 7 3.1 58.42 all 9.3 3.89 58.84 Slight bubbling 11.1 3.98 59.31 Bubbling 13.5 4.04 59.71 Fairly turbulent bubbling 16.3 4.1 60.45 Turbulent bubbling 19.7 4.11 61.02 Highly turbulent bubbling 17.2 4.11 60.61 Highly turbulent bubbling 14.4 4.11 60.15 Turbulent bubbling 12.2 4.03 59.57 Just above even mixing 10.1 3.97 58.72 Bubbling Unit Operations ChE-381 Group No. 1 p. 28 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 T (°F) 100 99 97 94 88 108 105 105 104 104 103 Flow Through Fluidized Beds University of Illinois 8.7 3.9 58.46 7.1 3.5 58.32 0 -0.62 58.32 Slight bubbling Stable, surface not bubbling at all Stable, surface not bubbling at all 98 94 94 Table 9. Density of Air Calculation Pressure (kPa) 101.325 101.325 101.325 R constant (kPa*m3/kmol*K) Temperature (+/- 0.5 K) rho (kmol/m^3) 8.314 8.314 8.314 Substance Large Sand Small Sand Silica 298 296 311 0.0409 0.0411 0.0392 Table 10. Void Fraction Calculation Dry Volume (+/- Water Volume(+/Combined 0.1 mL) 0.1 mL) Volume(+/- 0.1 mL) 5 15 18 5.5 16 18.9 8.8 15.5 20.4 Density of Air (kg/m^3) 1.19 1.19 1.14 Void Fraction (dimensionless) 0.40 0.47 0.44 Table 11. Superficial Velocity Calculation Substance Large Sand/Trial 1 Large Sand/Trial 2 Small Sand/Trial 1 Small Sand/Trial 2 Substance Silica / Unheated Silica / Heated Flowrate (L/s, +/1%) 1.83 1.88 1.36 1.43 Flowrate (ft^3/min, +/- 1%) 37.5 27.4 Flowrate (m^3/s) Area (m^2) 0.00183 0.00188 0.00136 0.00143 0.0408 0.0408 0.0408 0.0408 Superficial Velocity (m/s) 0.045 0.046 0.033 0.035 Flowrate (m^3/s) Area (m^2) Vs (m/s) 0.0177 0.0129 0.0403 0.0403 0.440 0.321 Table 12. Calculation Summary Density Density Particle Fluidization Superficial µ Void Fraction Substance of Bed of Air Diameter Velocity Velocity (Pa*s) (dimensionless) (kg/m^3) (kg/m^3) (m) (m/s) (m/s) Large Sand/Trial 1600 1.19 4.46E-04 1.96E-05 0.40 0.11 0.045 1 Large Sand/Trial 1600 1.19 4.46E-04 1.96E-05 0.40 0.11 0.046 2 Unit Operations ChE-381 Group No. 1 p. 29 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds Small Sand/Trial 1 Small Sand/Trial 2 Silica / Unheated Silica / Heated University of Illinois 1600 1.19 1.80E-04 1.96E-05 0.47 0.035 0.0333 1600 1.19 1.80E-04 1.96E-05 0.47 0.035 0.0350 2100 1.19 6.71E-04 1.94E-05 0.44 0.50 0.440 2100 1.14 6.71E-04 2.00E-05 0.44 0.48 0.321 9. Results For this lab, trials were run at two different starting bed heights of sand, each tested against two ranges of grain size, and two different temperatures of silica at equal starting bed heights and one range of grain sizes. The grain size ranges of sand were 63-297 microns and 297-595 microns. The range of particle sizes for the silica was 500-841 micrometers. For each trial, the data collected included bed height, pressure drop, airflow rate, and temperature for the silica trials. The data collected is best represented in figures 3, 4, and 5. Figure 3 shows the relation between airflow rate and the overall pressure drop in the column for a bed height of approximately 10.5 cm, and both sand sieve ranges. The portion where pressure drop starts levels off is the point of minimum fluidization. We can see that for the large sieve size, minimum fluidization occurs at a flow rate of approximately 85% of 2.2L/s. Similarly, for the small sieve size, minimum fluidization occurs at a flow rate of approximately 65% of 2.2L/s. Fluidization occurs when the superficial velocity equals the minimum fluidization velocity, and superficial velocity is directly proportional to the flowmeter reading. Tables 3 and 5 show that fluidization for the smaller bed height occurs at 83.3% and 61.8% of 2.2L/s, for large and small sieve sizes, respectively. Unit Operations ChE-381 Group No. 1 p. 30 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 1.7 1.45 Pressure Drop (in H2O) 1.2 0.95 0.7 0.45 Large Sand Size 0.2 Small Sand Size -0.05 0 10 20 30 40 50 60 70 80 90 100 -0.3 -0.55 -0.8 Flowmeter Reading, percentage of 2.2 L/S Figure 3. Pressure drop vs flowmeter reading for large bed heights of sand. Superficial velocity increases as flowmeter reading increases. Fluidization occurs when the slope of the graph levels off. Figure 4 shows the same correlation of air flow rate to pressure drop for the silica column at both room temperature and the increased temperature average of about 103° F. The data displayed below shows this correlation for both increasing and decreasing flow rates. For cool silica, fluidization occurred at 27% of 139 CFM, and for hot silica, fluidization occurred at 16.3% of 139 CFM. Unit Operations ChE-381 Group No. 1 p. 31 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 4.3 3.79 Pressure Drop (in H2O) 3.28 2.77 2.26 1.75 Cool Silica 1.24 Hot Silica 0.73 0.22 -0.29 0 5 10 15 20 25 30 35 40 -0.8 Flowmeter Reading, percentage of 139 CFM Figure 4. Pressure drop vs flowmeter reading for silica. Silica bed was heating to approximately 103°F. The pressure drop was the same magnitude for both cases. Figure 5 illustrates the correlation between airflow rate and bed height of the larger sand particles from both increasing and decreasing flow rates. When the experiment began, the bed of sand was 47.13 centimeters above the workbench that apparatus was mounted on. Increases in flow rate lead to an increase of the bed height and a decrease in the flow rate leads to a decrease in bed height. This makes sense since the air flowing through the bed applies a force in the vertical direction causing the bed height to change. This graph is representative for all tested scenarios. Unit Operations ChE-381 Group No. 1 p. 32 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 50 49.65 49.3 Bed height (cm) 48.95 48.6 48.25 47.9 47.55 Large Sand Size 47.2 46.85 46.5 0 10 20 30 40 50 60 70 80 90 100 Flowmeter Reading, percentage of 2.2 L/S Figure 5. Bed height vs flowmeter reading for large sand size at the small bed height. Bed height increases with increasing flow rate. This figure is representative for all tested scenarios. 10. Discussion A fluidized bed can be created by a gas flowing through a bed of solid particles that are in some kind of vessel, for our experiment inside a column. Under the appropriate conditions the gas will cause the solid to begin to behave as a fluid and when this occurs the bed is fluidized. Fluidized beds are useful because they create a large contact surface area between the solid and gas, which allows for an increase in the overall heat or mass transfer. Some of uses of this technique in chemical engineering are in the processes of fluid catalytic cracking and fluidized bed combustion, both of which are widely used in the energy industries today. For this experiment we had two columns, one filled with sand and the other with silica. For the silica we only used one height and grain size range, 500-841 microns, but varied the Unit Operations ChE-381 Group No. 1 p. 33 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois temperature to determine any effect this has on rate of fluidization. For sand we used two grain size ranges, 63-297and 297-595 microns, at two different bed heights. We then measured the change in pressure as the flow rate of the air was increased until fluidization occurred, and then decreased back down to zero. For the silica column, in addition to pressure drop, the temperature was also recorded at every flow rate. We also took note of any observations regarding the surface of the silica or sand bed as the flow rate of air was changed. The void fraction was also calculated for silica and both grain size ranges of sand. For every tested scenario, as the flow rate was increased, the height of the bed also increased, because the air flowing through the bed forced the solid to move vertically. With respect to the silica column, we can see that an increased temperature decreases the flow rate required to cause fluidization, and, by extension, the minimum fluidization velocity. At high temperatures, because of this decrease in flow rate, the pressure changes at a faster rate for hot silica than it changes for cooler silica. This temperature effect on minimum fluidization velocity cannot be explained as a viscosity effect, as minimum fluidization velocity is inversely proportional to viscosity, and the viscosity of air increases with increasing temperature, implying that minimum fluidization velocity should increase with increasing temperature, not the opposite as shown in the experiment. The temperature effect on minimum fluidization velocity could be due to convective heat transfer between the air and heated silica: the hot silica at the top of the bed transfers heat by convection to the air around it, which rises due to its own buoyancy. If the velocity of this convective hot air is non-negligible with respect to the forced airflow through the bed, it could be a sizable effect on minimum fluidization velocity. With respect to the sand column, using smaller grain sizes of sand increased the magnitude of the pressure drop at fluidization, as well as decreasing the necessary flow rate Unit Operations ChE-381 Group No. 1 p. 34 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois required to fluidize the bed. The rise in the magnitude of the pressure drop is unexpected, as the pressure drop is directly proportional to the amount of material that is not void space. It was shown that the smaller grains of sand had more void, implying less non-void space, implying a lesser pressure drop. For this increased pressure drop to occur, that would mean the bed of smaller sand grains must have had a higher density. The decrease in the minimum fluidization velocity is expected however, as minimum fluidization velocity is directly proportional to the square of the equivalent spherical diameter of the bed grains, and, as such, smaller grains decrease the minimum fluidization velocity. The major reason for errors in this experiment derives from a lack of true knowledge of the equivalent spherical diameter of the bed grains. Minimum fluidization velocity is directly proportional to the square of the equivalent spherical diameter of the bed grains. In this experiment, it is assumed that the simple average of the sieve size is a good approximation for this equivalent spherical diameter, but this could be problematic, as having more small grains than large grains would mean the true equivalent diameter would be less than the simple average. Similarly, having more large grains than small grains would mean the true equivalent diameter would be more than the simple average. Other reasons for error in our data and calculations came from the fact that the flow rate of the air fluctuates, due to the source being shared, so we had to take approximate values for the flow rates in our calculations. The air flow meters were extremely sensitive so we could not change our flow rates at a constant interval, which would have helped the usefulness of the calculated values. Another factor that contributed to the error was the heights of the beds of sand and silica, which were measured with a meter stick, from the outside of the column. A more accurate way of measuring the heights of the beds would include graduations being marked on Unit Operations ChE-381 Group No. 1 p. 35 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois the column, perhaps with a black marker. There was also some error associated with the beds themselves. Since we had air flowing through the beds the heights changed and while the change was only slight with low flow rates at higher flow rates the height changed dramatically, making it difficult to get an accurate value for the height of the bed. The turbulent mixing associated with fluidization also changed the distribution of mass between any two points in the column. 11. Error Analysis Each piece of equipment contributes to uncertainties in the measured data. These uncertainties affect the calculations in such a way that causes uncertainties in the results produced. The uncertainty in the results is calculated by propagation of errors from the measured uncertainties. The uncertainties associated with each piece of equipment are as follows: The airflow used for silica is measured with a flow meter that reads in percentage of 139.0 SCFM with markings at each 1% interval. Since the flow meter level remained constant throughout the experiment, one extra significant digit could be approximated which would create an uncertainty of 0.1%. The air flow meter used for sand reads in a percentage of 2.2 liters per second in intervals of 1%. As with the silica side air flow meter, one extra significant digit could also be approximated to an uncertainty of 0.1% of max flow. The manometer used for measuring the difference in pressure reads in intervals of 0.1 inches of water. Since the level of water in the manometer remained level at each flow rate, the last digit can accurately be measured to an uncertainty of 0.01 inches of water. The digital thermometer used to measure the temperature in the silica column only reads in integer values. We read the values in Fahrenheit because the scale is more sensitive than the Celsius scale; however, since the first decimal is rounded off, each reading has an uncertainty of 0.5° F. The height of material in each column was measured using a meter stick, which reads in increments of 1 mm. The height of the column could not be read as accurately due to bubbling and mixing of material at higher flow rates which caused the uncertainty of each measurement to be 0.25 cm at high flow rates. Unit Operations ChE-381 Group No. 1 p. 36 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds Equipment Air Rotameter (Silica) Air Rotameter (Sand) University of Illinois Table. 13 - Error Analysis Units Uncertainty SCFM (Standard ± 0.1 % of Cubic Feet per 139 scfm Minute) L/s (cubic ± 0.1% of centimeters per 2.2 L/s second) Monometer Inches of Water ± 0.01 inches H2O Digital Thermometer °F or °C ± 0.5 °F or °C Bed height measurements with meter stick cm (centimeters) ± 0.25 cm Reason Lowest readable increment is 1%. Last digit approximated. Lowest readable increment is 1%. Last digit approximated. Lowest readable increment is 0.1 inch. Last digit approximated. Only reads integer values which are rounded off to the nearest value Height of material fluctuates at higher flow rates 12. Conclusion Fluidized beds provide a large surface area for contact between solids and a liquid or a gas that is conducive for heat and mass transfer providing for an environment where nearly uniform temperatures can be maintained in the reactor even with highly exothermic reactions. This is important because hot or cold spots may develop in beds where there is a temperature gradient or the temperature is not uniform. These hot spots can cause equipment failure and product degradation while the cold spots decrease the efficiency of the reaction. A fluidized bed also provides uniform mixing, which is important for product quality and efficiency. Consequently, the study of fluidized beds is valuable to the chemical engineering field. In this experiment we analyzed the effects of grain size and temperature to determine the minimum fluidization velocity (Vf ). The trials were run at two different starting bed heights of sand each for two ranges of grain size, and two different temperatures of silica at equal starting bed heights and one range of grain sizes. The grain size ranges used for sand were 63-297 µm Unit Operations ChE-381 Group No. 1 p. 37 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois and 297-595 µm. For the silica the particle size ranged from 500-841 µm. For the 297-595 micrometer range sand the bed heights were 7.31 cm and 10.19 cm and for the 63-297 micrometer range the bed heights were 6.63 cm and 11.10 cm. Finally for the silica we used bed heights of 19.15 cm and 18.34 cm. The temperature range for the silica trials was 69-78˚F for the colder trial and 88-100˚F for the warmer trial. The void fraction was also calculated for the large sand (0.40), small sand (0.47), and silica (0.44). For each trial, the data collected included bed height, pressure drop, airflow rate, and temperature for the silica trials. As expected when the flow rate increased the bed height also increased. As the flow rate increased the sand and silica exhibited different behaviors. At very low flow rates there was no appreciable impact on the silica and sand. As the flow rate gradually increased the bed began to bubble and eventually reached the minimum fluidization velocity. Beyond this point the beds began to exhibit turbulent behavior with severe bubbling and mixing. The minimum fluidization velocity was experimentally determined to be 0.45 and 0.46 m/s for the large sand trials, 0.0333 and 0.0350 m/s for the small sand trials, and 0.44 and 0.321 m/s for the silica trials. These values are determined by identifying the point in the flow rate vs pressure drop curves where the graph first flattens out; this is the point where the derivative is equal to zero. Therefore as grain size decreases, the minimum fluidization velocity also decreases. This result makes sense because a fluid is essentially composed of several tiny particles condensed together. From the silica trials it can be inferred that as bed temperature increases the minimum fluidization velocity decreases; that is hotter beds fluidize faster. 13. References 1. Flow Through Fluidized Bed Lab Manual. 2. Fluidized Bed Reactor. http://en.wikipedia.org/wiki/Fluidized_bed_reactor Unit Operations ChE-381 Group No. 1 p. 38 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 3. Material Safety Data Sheet: Sand. Fisher Scientific. http://fscimage.fishersci.com/msds/09890.htm 4. Material Safety Data Sheet: Silica Gel Desiccant. Fisher Scientific. http://www.atmos.umd.edu/~russ/MSDS/silicagel28200.html 5. Fogler, Scott H. Elements of Chemical Reaction Engineering. 4th Ed. Boston: Pearson. 2006. Unit Operations ChE-381 Group No. 1 p. 39 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 14. Appendix I In this appendix, it will be shown how the minimum fluidization velocity and interstitial velocity for the first trial of the large sand size was calculated. Starting from the minimum fluidization velocity equation: Vf ( p f ) gD2p 150 ( 3 1 ) Where: Vf , = Minimum fluidization velocity (m/s) = Density of the bed (kg/m3) = Density of the fluid, air (kg/m3) g = gravitational constant (9.81 m/s2) Dp = Equivalent spherical diameter of the bed particles (m) μ = Dynamic viscosity of the fluid, air (Pa-s) ε = Void fraction of the bed (dimensionless) , Dp, μ, and ε are our unknowns of interest. We will start with void fraction ε. Void fraction is defined as: Void Volume Dry Volume From our preliminary data for the large sand size, 5 mL of sand were mixed with 15 mL of water in a graduated cylinder, yielding an 18 mL solution. Thusly, two mL of the water added filled the void space in the sand, so: Unit Operations ChE-381 Group No. 1 p. 40 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois (20 18) 0.4 5 From the void fraction, we can obtain the density of the bed. We postulate that sand is simply made up of silicon dioxide (SiO2) particles and void space, so: p s (1 ) Where: = Density of the bed (kg/m3) s = Density of pure SiO2 (kg/m3) ε = Void fraction of the bed (dimensionless) Knowing that pure SiO2 has a density of 2634 kg/m3, and using our previously found void fraction: p 2634 * (1 .4) p 2634 * 0.6 kg p 1580.4 1600 3 m , the density of the air, can be easily obtained from the ideal gas law: P f RT Where: P = Ambient Pressure (101.325 kPa) = Density of the air (kmol/m3) R = Gas constant (8.314 kPa*m^3/kmol*K) T = Absolute Temperature (298 K) Unit Operations ChE-381 Group No. 1 p. 41 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds Solving for University of Illinois : (101.325) 0.0409 kmol f (8.314)( 298) m3 0.0409 kmol 29 kg 1.19 kg * 3 m kmol m3 Dp is taken as the simple average of the size of the sieve used. This trial corresponded to a sieve range of 297-595 microns. Thusly: (595 297) * 10 6 m 2 Dp 4.46 *104 m Dp μ, the viscosity of the air, is linearly interpolated between two known values of μ at 250K and 300K. μ for a temperature of 298K is: 1.9632 *105 Pa* s We have solved for all of our unknowns and can find Vf, the minimum fluidization velocity: (1600 1.19) * (9.81) * (4.46 *104 )2 0.43 Vf ( ) 150(1.9632 *105 ) 1 0.4 0.11 m Vf s This will be compared to the interstitial velocity, calculated using the flow rate, Q. Minimum fluidization occurs when the pressure drop across the fluidized bed stops increasing. In this trial, the flowmeter read 83.3% of 2.2 L/s when the pressure drop stabilized. As such: Q 83.3 * 2 .2 100 Unit Operations ChE-381 Group No. 1 p. 42 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 1.83 L 1 m3 0.00183 m3 Q * s 1000 L s From this flow rate, we can calculate the interstitial velocity by dividing the flow rate, Q, by the cross sectional area, A. Knowing the diameter of the column is 0.114 m: A 0.0408 m 2 Q 0.00183 0.045 m Vs A 0.0408 s Unit Operations ChE-381 Group No. 1 p. 43 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 15. Appendix II The lab group members and their contributions are presented below 13 hrs 45 min Krista Sutton Time Spent in Lab Section 1 – WP&C 2 – Abstract 3 – Introduction 4 – Theory 5 – Apparatus 6 – Materials and Supplies 7 – Procedure 8 – Data Tabulation/Graphs 9 – Results 10 – Discussion 11 – Error Analysis 12 – Conclusion 13 – References 14 – Appendix I 4 hrs Time Spent 15 min 15 min 30 min 45 min 4 hrs 30 min 30 min 1 hr 45 min 30 min 15 min 15 min 15 min 0 hrs 0 hrs Unit Operations ChE-381 Group No. 1 p. 44 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Comments Review Review Review Review Writing initial Drat Writing initial Draft Review Reformatting Tables Review Review Review Review Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 16 hrs 0 min Jake Biberstein Time Spent in Lab Section 1 – WP&C 2 – Abstract 3 – Introduction 4 – Theory 5 – Apparatus 6 – Materials and Supplies 7 – Procedure Time Spent 0 hrs 15 min 1 hr 2 hrs 0 hrs 0 hrs 0 hrs 8 – Data Tabulation/Graphs 5 hrs 9 – Results 10 – Discussion 11 – Error Analysis 12 – Conclusion 13 – References 14 – Appendix I 4 hrs 1 hr 30 min 0 hrs 15 min 15 min 1 hr 45 min Unit Operations ChE-381 Group No. 1 p. 45 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Comments Group Section Edited Mike’s First Version Edited Mike’s First Version Did the master Excel file which contained all graphs, calcs, tables… Edited Russ’s First Version Edited Stan’s First Version Group Section Provided the things I used Wrote the section Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 10 hrs 0 min Stanley Das Time Spent in Lab Section 1 – WP&C 2 – Abstract 3 – Introduction 4 – Theory 5 – Apparatus 6 – Materials and Supplies 4 hrs Time Spent 0 hrs 30 min 0 hrs 0 hrs 0 hrs 30 min 7 – Procedure 0 hrs 8 – Data Tabulation/Graphs 1 hr 9 – Results 10 – Discussion 11 – Error Analysis 0 hrs 3 hrs 30 min 12 – Conclusion 30 min 13 – References 14 – Appendix I 0 hrs 0 hrs Unit Operations ChE-381 Group No. 1 p. 46 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Comments N/A Helped write this section with the rest of the group N/A N/A N/A Reviewed this section for errors and typos N/A Input all data collected into excel spreadsheets, increased font size of axes, legend. Collected data. N/A Wrote first draft of this section Wrote first draft of this section Reviewed this section for errors and typos N/A N/A Fall 2009 10/22/2009 Flow Through Fluidized Beds Michael Czepizak Time Spent in Lab Section 1 – WP&C 2 – Abstract 3 – Introduction 4 – Theory 5 – Apparatus 6 – Materials and Supplies 7 – Procedure 8 – Data Tabulation/Graphs 9 – Results 10 – Discussion 11 – Error Analysis 12 – Conclusion 13 – References 14 – Appendix I University of Illinois 13 hrs 0 min Total Time Spend on this Lab 4 hrs Time Spent 10 min 10 min 2 hrs 30 min 2 hrs 30 min 0 0 0 0 10 min 10 min 0 3 hrs 10 min 10 min Unit Operations ChE-381 Group No. 1 p. 47 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Comments Reviewed the section Reviewed Wrote this section Wrote this section Reviewed this section Reviewed this section Wrote this section Add references Reviewed section Fall 2009 10/22/2009 Flow Through Fluidized Beds Jeff Umbach Time Spent in Lab Section 1 – WP&C 2 – Abstract 3 – Introduction 4 – Theory 5 – Apparatus 6 – Materials and Supplies 7 – Procedure 8 – Data Tabulation/Graphs 9 – Results 10 – Discussion 11 – Error Analysis 12 – Conclusion 13 – References 14 – Appendix I University of Illinois 13 hrs 10 min Total Time Spend on this Lab 4 hrs Time Spent 45 min 10 minutes 30 min 1 hr 30 min 20 min 4 hrs 10 min 15 min 15 min 30 min 10 min 20 min 15 min Comments Revised and proofread. Formatted and proofread Revised and proofread. Edited, revised, and proofread. Revised and proofread. Revised and proofread. Wrote original and revised as issues were found during the lab experiment. Proofread Proofread and fixed grammar. Proofread and fixed grammar. Revised and proofread. Fixed grammar. Formatted, revised, and proofread. Tracked down some references. Proofread Unit Operations ChE-381 Group No. 1 p. 48 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Fall 2009 10/22/2009 Flow Through Fluidized Beds University of Illinois 10 hrs 55 minutes Russ Boyer Time Spent in Lab Section 1 – WP&C 2 – Abstract 3 – Introduction 4 – Theory 5 – Apparatus 6 – Materials and Supplies 7 – Procedure 8 – Data Tabulation/Graphs 9 – Results 10 – Discussion 11 – Error Analysis 12 – Conclusion 13 – References 14 – Appendix I 4 hrs Time Spent 30 min 20 min 30 min 30 min 15 min 15 min 30 min 45 min 1 hr 30 min 15 min 1 hr 15 min 10 min 10 min 0 hrs Unit Operations ChE-381 Group No. 1 p. 49 Biberstein, Boyer, Czepizak, Das, Sutton, Umbach Comments Writing and revisions Writing and revisions Proofreading and editing Proofreading and editing Gathering apparatus specifications Gathering specifications Editing and revisions Data gathering and reduction Wrote initial draft Proofreading and editing Writing and proofreading Proofreading and editing Gathering references Fall 2009 10/22/2009
![LAB 4 FB Safety [BH]](http://s3.studylib.net/store/data/007109339_1-10edf2f99cf9e3f5eb5770ce96c065cf-300x300.png)