Enzyme Catalysis II - The Young Scientist Program
advertisement

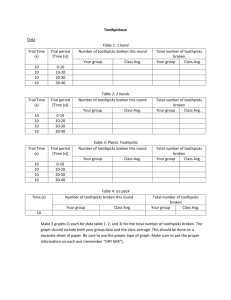
The Young Scientist Program http://ysp.wustl.edu Washington University Medical School Funding provided by The Leon Lowenstein Foundation Enzyme Catalysis II: the Enzyme “Toothpickase” Chemistry Teaching Team References: Adapted from Ms. Foglia’s AP Biology lab course at Division Ave High School, Levittown, NY. See http://explorebiology.com/apbiology/. Main Concepts: Chemical reaction rates Enzymes and substrates Module Overview: Enzymes speed up the rate of a chemical reaction without being used up during the reaction. A substrate is the molecule that an enzyme acts on. Enzymes can link substrates together, break substrates apart, or change the shape of a substrate. We will do some experiments with a model enzyme to understand how an enzyme makes it possible for a reaction to proceed more quickly. Materials: Toothpicks Safety clips Stopwatch Graph paper Ice water Safety: students do not need to use gloves or goggles for protection in this experiment. Be careful of splinters when touching broken toothpicks. Methods/Protocol: We will do an activity to simulate how substrate concentration and temperature affect enzyme function. In this activity, let us assume: one person’s fingers are the enzyme “toothpickase” toothpicks are the substrate of toothpickase toothpickase catalyses the breakdown of an intact toothpick into 2 pieces (it “digests” the toothpick). I. Rate of product formation in an enzyme-facilitated reaction 1. Place 40 toothpicks in a shallow dish. The Young Scientist Program http://ysp.wustl.edu Washington University Medical School Funding provided by The Leon Lowenstein Foundation 2. One student will be the timer: use the stopwatch to record the time required to complete the reaction. One student will be the recorder: count how many toothpicks have been broken in a given time period. One student will be the enzyme: break the toothpicks without looking, using only one hand. 3. the experiment is conducted in 20-second intervals for 60 seconds. The timer calls out start and then notes each 20-second interval. The recorder tallies the number of toothpicks in each interval. time (seconds) # toothpicks broken rate toothpicks/second 0 20 40 60 4. graph the numbers from the table with time on the x axis and the number of toothpicks broken on the y axis. Discussion Questions: 1. What is the overall rate of enzyme action in toothpicks per second? 2. Is the rate constant during each interval of the experiment? 3. What environmental factors do you think could influence this reaction rate? II. Effect of substrate concentration on reaction rate 1. As before, we will need three volunteers to be the timer, recorder, and enzyme. 2. Mix in 40 paper clips with the number of toothpicks listed below. The paperclips represent a “solvent” in which the toothpicks are “dissolved”. The enzyme cannot break the paperclips, but must continue searching for toothpicks if he or she picks one up accidentally. The enzyme will have 20 seconds to break as many toothpicks as possible. # paper clips 40 40 40 40 # toothpicks 50 30 10 5 toothpick/paper clip ratio #broken 1.2 Rate toothpicks/second The Young Scientist Program http://ysp.wustl.edu Washington University Medical School Funding provided by The Leon Lowenstein Foundation 3. Graph the reaction rate versus the toothpick/paper clip ratio. Discussion Questions: 1. how did the “solvent” paper clips affect the reaction rate? The solvent decreased the reaction rate by decreasing how often the enzyme found a substrate to react on. III. Effect of temperature on reaction rate 1. As before, we will need three volunteers to be the timer, recorder, and enzyme. 2. Select 10 toothpicks. Time how long it takes the enzyme to break the 10 toothpicks as quickly as he or she can (without looking). Calculate the rate of enzyme action in toothpicks per second. 3. Place the enzyme’s hand in a pail of ice water for 30 seconds. Repeat step 2. Discussion Questions: 1. What was the effect of cold on the enzyme? The cold ice bath is likely to slow down the enzyme. This is certainly the case for most naturally occurring enzymes. 2. What do you think would happen if the temperature were increased? What if the temperature were increased to 100C (boiling)? The reaction rate might increase if the temperature were increased slightly above room temperature. However, increasing the temperature to boiling would stop the reaction of most enzymes because enzymes are proteins, and become denatured at high temperatures. 3. What is the optimal temperature for enzymes that function in the human body? The body’s physiological temperature is 37C (98.6F) and this is the optimal temperature for the enzymes within our bodies to function. The Young Scientist Program http://ysp.wustl.edu Washington University Medical School Funding provided by The Leon Lowenstein Foundation I. II. The Young Scientist Program http://ysp.wustl.edu Washington University Medical School Funding provided by The Leon Lowenstein Foundation