Experiment B. Isolation of Trimyristin from Nutmeg
advertisement

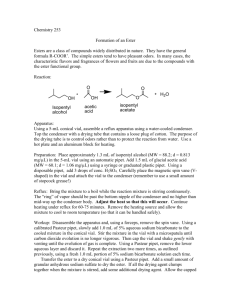
Organic Chemistry I Lab CHEM 2211L (Fall 2008) School of Science & Technology Georgia Gwinnett College Experiment B. Isolation of Trimyristin from Nutmeg ______________________________________________________ 1. EXPERIMENTAL OBJECTIVES: a. Use extraction techniques to isolate a natural product (trimyristin) from nutmeg. b. Characterize and analyze the product. c. Present, analyze, and discuss results in a written report. 2. SPECIAL INSTRUCTIONS: a. Before you come to the lab to begin the experiment, you should complete the lab report sections in your lab notebook up to procedures as described in the “Lab notebook guidance” posted on the course web page. Do the best you can in finding chemical information for the UTORP via web searches such as Google. The sections on procedures, data, and observations must be recorded directly into your laboratory notebook as you actually do the experiment. You must come to the lab prepared to immediately set up equipment, obtain reactant chemicals, and begin the reaction. Although the reflux procedure only requires 0.5 hours, the subsequent cooling, solvent evaporation and recrystallization steps can be lengthy. If you are not prepared, you will not be able to isolate your product by the end of the first lab session. b. Chemicals and solvents: (1) diethyl ether, [60-29-7] (2) 2-propanone (acetone), [67-64-1] c. Now that you are in the second organic lab and you are using your lab notebook, you will notice that there are very few specified tasks or questions for you to address. Therefore, you should develop a list of implied tasks based on the nature of the experiment and address these in your lab report. d. You will turn in the original pages of your notebook at the end of the experimental portion of the lab (at the end of hour 6). You will then complete the remainder of the report (calculations, analysis, discussion, etc) outside of class and turn in the remaining pages at the beginning of the next class period. Remember, once you complete the experimental portion, you cannot go back and write observations into you notebook at a later time---it is a real time journal. 3. PROCEDURES: WATCH FOR HOT SURFACES--USE EXTREME CARE WHEN POSITIONING EQUIPMENT ON YOUR HOT PLATE! You will be pressed for time in this lab and should Final Draft Page 1 of 3 3/6/16 Organic Chemistry I Lab CHEM 2211L (Fall 2008) School of Science & Technology Georgia Gwinnett College get to the point of isolating your product during the first three-hour session. The second session is for characterization of your product. Apparatus 1. You will do the first part of the lab in your hood. The desired temperature for your sand bath is about 45-50oC (not very hot). It is better to heat gradually and be a little low of the target temperature than to be far above the target temperature. (Think about the boiling points of the various reagents.) 2. Dry-fit your experimental equipment, to include the magnetic stir bar (use the conical stir vane), before you get reagents. Use the 10 mL round bottom flask as your reaction vessel instead of the conical vial. Once you’ve assembled your apparatus, ensure that cold water is flowing through the condenser at an appreciable rate before heating. Make sure the condenser hoses are secure and the exhaust hose is in the sink drain. 3. When preparing your Pasteur filter pipet (G&M Fig 2.9, p. 34 and Fig 2.51, p. 68), use the smallest bit of cotton possible. Experimental 4. Reflux gently. If you see vapor above the condenser, your sand bath is too hot. Reflux means that the reaction mixture is gently heated at its boiling point while the COLD water cooled condenser allows continuous return of the volatile materials to the reaction flask. 5. After refluxing and cooling to room temperature, transfer your ethereal solution to a conical vial, not the Craig tube. When transferring the ethereal solution via Pasteur filter pipet into the conical vial, use care not to suck up solids from the reaction vial or the filter pipet will clog up. Fill the Pasteur pipet with about 1 inch of Na2SO4 on top of the cotton plug before transferring solution. This will help remove any water that is causing the solution to be cloudy in appearance. (This may be done twice in order to remove cloudiness.) 6. When evaporating the ethereal solution, warm the conical vial very gently in your sand bath in the hood. Do not use a boiling chip. Be careful not to heat too much or your material will bump right out of the vial and you will lose it forever! You want to heat until the solvent is gone and an oily residue remains. 7. After addition of the acetone to the residue, apply only a slight bit of heat. Watch for and avoid boiling/bumping. Work-Up 8. Exercise care when centrifuging the Craig tube, as the product may be deposited on the tube surface. Final Draft Page 2 of 3 3/6/16 Organic Chemistry I Lab CHEM 2211L (Fall 2008) School of Science & Technology Georgia Gwinnett College 9. After centrifuging is complete, place crystals on a piece of filter paper on a watch glass, and spread out the crystals to aid in drying. Analysis 10. Calculate % recovery and obtain a melting point for your product. If time permits and you choose to do so, work with your instructor to take an IR of your product and analyze it. 11. Disposal of chemical and solid waste. MAKE SURE YOU REMOVE THE MAGNETIC SPIN VANE FROM YOUR REACTION VIAL BEFORE CLEANING, OTHERWISE IT WILL BE LOST IN THE WASTE BOTTLE. Discard any glass pipettes you may have used into the broken glass/glass waste container. Final Draft Page 3 of 3 3/6/16