Chemistry 253
advertisement

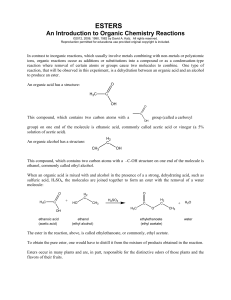
Chemistry 253 Formation of an Ester Esters are a class of compounds widely distributed in nature. They have the general formula R-COOR’. The simple esters tend to have pleasant odors. In many cases, the characteristic flavors and fragrances of flowers and fruits are due to the compounds with the ester functional group. Reaction: O OH Isopentyl alcohol O OH O acetic acid isopentyl acetate H2O Apparatus: Using a 5-mL conical vial, assemble a reflux apparatus using a water-cooled condenser. Top the condenser with a drying tube that contains a loose plug of cotton. The purpose of the drying tube is to control odors rather than to protect the reaction from water. Use a hot plate and an aluminum block for heating. Preparation: Place approximately 1.3 mL of isopentyl alcohol (MW = 88.2; d = 0.813 mg/µL) in the 5-mL vial using an automatic pipet. Add 1.5 mL of glacial acetic acid (MW = 60.1; d = 1.06 mg/µL) using a syringe or graduated plastic pipet. Using a disposable pipet, add 3 drops of conc. H2SO4. Carefully place the magnetic spin vane (Vshaped) in the vial and attach the vial to the condenser (remember to use a small amount of stopcock grease!) Reflux: Bring the mixture to a boil while the reaction mixture is stirring continuously. The “ring” of vapor should be past the bottom nipple of the condenser and no higher than mid-way up the condenser body. Adjust the heat so that this will occur. Continue heating under reflux for 60-75 minutes. Remove the heating source and allow the mixture to cool to room temperature (so that it can be handled safely). Workup: Disassemble the apparatus and, using a forceps, remove the spin vane. Using a calibrated Pasteur pipet, slowly add 1.0 mL of 5% aqueous sodium bicarbonate to the cooled mixture in the conical vial. Stir the mixture in the vial with a microspatula until carbon dioxide evolution is no longer vigorous. Then cap the vial and shake gently with venting until the evolution of gas is complete. Using a Pasteur pipet, remove the lower aqueous layer and discard it. Repeat the extraction two more times, as outlined previously, using a fresh 1.0 mL portion of 5% sodium bicarbonate solution each time. Transfer the ester to a dry conical vial using a Pasteur pipet. Add a small amount of granular anhydrous sodium sulfate to dry the ester. If all the drying agent clumps together when the mixture is stirred, add some additional drying agent. Allow the capped solution to stand for 10-15 minutes. Transfer the dry ester with a Pasteur pipet into a 3mL conical vial while leaving the drying agent behind. Distillation: Add the clean magnetic spin vane to the dry ester. Clamping the glassware, assemble a distillation apparatus using a Hickman still and a water-cooled condenser and thermometer with adapter on top of a hot plate with an aluminum heating block. Continue the distillation until only one or two drops of liquid remain in the distilling vial. Note the temperature range where material is being collected. If the Hickman head fills before the distillation is complete, you will need to empty it using a Pasteur pipet. Transfer the distillate to a pre-weighed vial. Determination of yield: Weigh the product and calculate the percentage yield of the ester. Report the boiling range of the ester. What common “flavoring” does the ester resemble? Include a reagents chart (usual) in your lab report.