Introduction - Cedar Crest College
advertisement

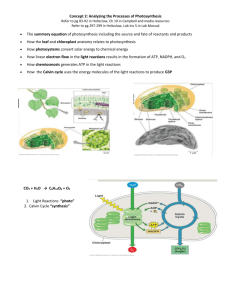
Identifying Photosynthetic Reactants and Products • Photosynthesis, the biochemical process by which plants capture energy from sunlight and store it in carbohydrates, sugars, and starch, is the very basis of life on Earth. • By the 1800s, scientists understood that there were three principal ingredients for photosynthesis: water, carbon dioxide, and light. • There are two products: carbohydrates and oxygen. (See Figure 8.1.) • The water, which comes primarily from the soil, is transported through the roots to the leaves. • The CO2 is taken in from the air through stomata, or pores, in the leaves. • Light is an absolute necessity to produce oxygen and carbohydrates. • By 1804, scientists summarized the overall chemical reaction of photosynthesis: CO 2 + H2O + light energy sugar + O2 • More recently, using water and carbon dioxide labeled with radioactive isotopes of oxygen, it has been determined that the actual reaction is 6 CO2 + 12 H2O C6H12O6 + 6 O2 + 6 H2O. • Water appears on both sides of the equation because water is both used as a reactant and released as a product. (See Figure 8.2 and Animated Tutorial 8.1.) The Two Pathways of Photosynthesis: An Overview • Photosynthesis occurs in the chloroplasts of green plant cells and, like metabolism, is a result of many steps—not just a single step. • When looked at as a whole, it can be separated into two different pathways. • The first pathway is called the light reaction and is driven by light energy that is captured by chlorophyll pigments. It produces ATP and the reduced electron carrier, NADPH + H+. • The second pathway, called the Calvin–Benson cycle, does not use light directly. It uses ATP, NADPH + H+, and CO2 to produce sugar. • The light reactions are mediated by molecular assemblies called photosystems. • These systems pass electrons from one molecule to another, and some of this flow is coupled to synthesis of ATP. • The pathway is referred to as photophosphorylation. Both NADPH + H+ and ATP are produced by the light reactions. • The Calvin–Benson cycle uses the energy stored in NADPH + H+ and ATP to fix CO2 into carbohydrates. • Note that although the Calvin–Benson cycle is sometimes referred to as the dark reactions; the cycle requires the products of the light reactions to run, and it stops in absence of light. The Interactions of Light and Pigments • Light is the source of the energy required to drive photosynthesis. Light behaves as both a particle and a wave • • • • • • Visible light is part of the electromagnetic radiation spectrum. It comes in discrete packets called photons. Light also behaves as if it were a wave. The amount of energy contained in a single photon is inversely proportional to its wavelength. Two things are required for photons to be active in a biological process: Photons must be absorbed by receptive molecules. Photons must have sufficient energy to perform the chemical work required. Absorbing a photon puts a pigment in an excited state • When a photon and a pigment molecule meet, one of three things happens: • The photon may bounce off the molecule. This is scattering. • The photon may pass through the molecule. This is transmission. • The photon may be absorbed by the molecule. This is excitation. • If absorbed, the photon disappears, but the energy it possessed can be neither created nor destroyed and is therefore acquired by the molecule. • The molecule is raised from its ground state to an excited state of higher energy. • The difference between the excited and the ground state is precisely equal to the energy of the absorbed photon. (See Figure 8.4.) • Different photons can have a wide range of wavelengths, and thus energies, associated with them. (See Figure 8.5.) • Molecules that absorb wavelengths in the visible range are called pigments. • When a beam of white light shines on an object, and the object appears to be red in color, it is because it has absorbed all other colors from the white light except for the color red. • In the case of chlorophyll, plants look green because they absorb green light less effectively than the other colors found in sunlight. Absorbed wavelengths correlate with biological activity • A given type of molecule can absorb radiant energy of only certain wavelengths. • If we plot the absorption of the compound as a function of wavelength, the result is an absorption spectrum. (See Figure 8.6a.) • Absorption spectra are good “fingerprints” of compounds. Sometimes an absorption spectrum contains enough information to enable us to identify an unknown compound. • If absorption results in an activity of some sort, then a plot of the effectiveness of the light as a function of wavelength is called an action spectrum. • Figure 8.6b shows the action spectrum of photosynthesis by Anacharis, a freshwater plant. Photosynthesis uses energy absorbed by several pigments • Chlorophylls are the most important pigments in photosynthesis. (See Figure 8.7.) • Plants have two predominant chlorophylls: chlorophyll a and chlorophyll b. These molecules differ slightly in their structure. • Both have a similar ring structure. • In the center of the chlorophyll ring is a magnesium atom. At the peripheral location of the ring is a long hydrocarbon tail that can associate with the hydrophobic region of the thylakoid membrane. • These chlorophylls absorb blue and red wavelengths, which are near the ends of the visible spectrum. • Other accessory pigments absorb photons that are intermediate in energy between the red and blue wavelengths and then transfer a portion of that energy to chlorophylls. • Examples of accessory pigments are the carotenoids, such as -carotene, which absorb photons in the blue and blue-green wavelength and appear deep yellow in color. • (See Videos 8.1 and 8.2.) Light absorption results in photochemical change • A pigment molecule enters an excited state when it absorbs a photon. • The excited state is unstable, and the molecule may return to the ground state. • When this happens, some of the absorbed energy is given off as heat and the rest is given off as light energy, or fluorescence. • When fluorescence occurs, no chemical work is done. • If fluorescence does not occur, the pigment molecule may pass some of the absorbed energy to other pigment molecules. • Pigments in the photosynthetic organisms are arranged into energy-absorbing antenna systems. (See Figures 8.7 and 8.8.) • In these systems, pigments are packed together and attached to thylakoid membrane proteins to enable the transfer of energy. • The excitation energy is passed to the reaction center of the antenna complex. • In plants, the pigment molecule in the center is always a molecule of chlorophyll a. • There are other chlorophyll a molecules in the antenna, but they absorb light at shorter wavelengths. Excited chlorophyll in the reaction center acts as a reducing agent for electron transport • The ground-state chlorophyll, symbolized by Chl, is not much of a reducing agent, but excited chlorophyll (Chl*) is a good one. • The reducing capability of Chl* is increased because excited molecules have electrons zipping around farther away from the nucleus. (See Figure 8.8.) • Less tightly held, the electron is more likely to be passed on in a redox reaction to an oxidizing agent. • Chl* can react with an oxidizing agent in a reaction such as: Chl* + A Chl+ + A–. • This is the first consequence of light absorption: Chlorophyll becomes a reducing agent. • The electron is passed on to an oxidizing agent. Chlorophyll then becomes a positively charged ion, which is missing an electron. • This redox reaction would not have occurred in the dark. The Light Reactions: Electron Transport, Reductions, and Photophosphorylation • The energized electron that leaves the activated chlorophyll in the reaction center immediately participates in a series of redox reactions. • The electron flows through a series of carriers in the thylakoid membrane, a process termed electron transport. • Two energy-rich products of the light reactions, NADPH + H+ and ATP, are the result. • Similar to the NAD+ found in cellular respiration, NADP+ is nicotinamide adenine dinucleotide phosphate. This compound couples the photosynthetic pathways. • Electron transport in the thylakoid membranes sets up a charge separation whose potential energy is captured by the chemiosmotic synthesis of ATP in a process called photophosphorylation. • There are two different systems for transport of electrons in photosynthesis. • The noncyclic electron transport produces NADPH + H+ and ATP and also produces the oxygen that we find in the atmosphere. (See Figure 8.9.) • Cyclic electron transport produces only ATP. Noncyclic electron transport produces ATP and NADPH • Two photosystems are required: • Photosystems are light-driven molecular units that consist of many chlorophyll molecules and accessory pigments bound to proteins in separate energy-absorbing antenna systems. • Photosystem I uses light energy to reduce NADP + to NADPH + H+. • Photosystem II uses light energy to oxidize water molecules, producing electrons, protons, and O 2. • The reaction center for photosystem I contains a chlorophyll a molecule called P700 because it best absorbs light at a wavelength of 700 nm. • The reaction center for photosystem II contains a chlorophyll a molecule called P680 because it best absorbs light at a wavelength of 680 nm. • To keep noncyclic electron transport going, both photosystems must constantly be absorbing light. • Details of the reactions: • Photons are absorbed by chlorophyll P 680 molecules. (See Figure 8.9.) • The P680-excited electrons transfer to the electron transport chain. • In photosystem I, P700 absorbs photons and becomes excited, which leads to the reduction of an oxidizing agent called ferredoxin and the production of P 700+. • The P700+ returns to the ground state by accepting electrons passed through the redox chain from photosystem II. + • In summary, noncyclic electron flow takes a molecule of water, four photons, one molecule each of NADP and ADP, and one Pi to make one molecule each of NADPH + H+ and ATP and a half molecule of O2. Cyclic electron transport produces ATP but no NADPH • Energy captured in the light reactions is stored in ATP and NADPH + H + to drive the Calvin–Benson cycle, which uses more ATP than NADPH + H+. • To keep balance, cyclic electron flow makes ATP without making NADPH + H+. • The process is called cyclic because the electron cycles back to the same P 700 molecule. • Water does not enter into the cyclic electron flow reactions, and no oxygen is released. • In cyclic electron flow, photosystem I acts on its own, without photosystem II. (See Figure 8.10.) • The P700 molecule, photosystem I’s reaction center chlorophyll, starts at ground state. • It absorbs a photon and becomes P 700*. • The P700* molecule then reacts with oxidized ferredoxin (Fdox to produce reduced ferredoxin (Fdred). • In contrast to noncyclic photophosphorylation, where Fd red reduces NADP+, Fdred passes its electron to another oxidizing agent, plastoquinone (PQ). • The PQ molecule passes the electron to the cytochrome complex. • The electron continues down a redox chain, pumping protons as it goes. • P700+, the chlorophyll that lost an electron, gets it back again from the last reducing agent at the end of the chain. • In summary, cyclic electron flow takes photons through chlorophyll molecules, passing excited electrons through a redox chain to produce ATP and some free energy as heat. Electron-deficit chlorophyll is restored and the process repeats. Chemiosmosis is the source of the ATP produced in photophosphorylation • ATP molecules are produced by a mechanism similar to that described in Chapter 7 for mitochondria. • The same type of chemiosmotic mechanism operates in photosynthesis, but is called photophosphorylation. (See Figure 8.11 and Animated Tutorial 8.2.) • High-energy electrons move through a series of redox reactions, releasing energy that is used to transport protons across the membrane. • Active proton transport results in the proton-motive force: a difference in pH and electric charge across the membrane. • In chloroplasts, the electron carriers in the thylakoid membrane are oriented so as to move protons into the interior of the thylakoid, and the inside becomes acidic with respect to the outside. • The ratio of H+ inside versus outside a thylakoid is usually 10,000 to 1, which is a difference of 4 pH units. • This difference in pH leads to the diffusion of H+ out of the thylakoid through specific protein channels, ATP synthases. • The ATP synthases couple the formation of ATP to proton diffusion back across the thylakoid membrane. Making Carbohydrate from CO2: The Calvin–Benson Cycle • The Calvin–Benson cycle is the second main pathway of photosynthesis. • Most of the enzymes used to make sugar from CO2 are found dissolved in the stroma of the chloroplast. • The cycle created by these enzymes does not use sunlight directly; it uses the energy captured in ATP and NADPH + H+ in the light reactions. • Thus the “dark” reactions require light indirectly and take place only in the presence of light. Isotope labeling experiments revealed the steps of the Calvin–Benson cycle • Radioactively labeled carbon in CO2 was used to identify the products generated by photosynthesis in the uni-cellular green alga, Chlorella. (See Figure 8.12 and Animated Tutorial 8.3.) • After a 30-second exposure to 14CO2, the cells were killed and carbohydrates and amino acids were extracted. • Many compounds, including monosaccharides and amino acids, contained 14C. • The exposure time to 14CO2 was shortened to just 3 seconds. • Only one compound was found labeled with 14C. • The compound was a three-carbon sugar called 3-phosphoglycerate (3PG). • Other products of the cycle were found by increasing the length of time of exposure in a stepwise manner until the whole pathway was revealed. • Finding a labeled three-carbon sugar was unexpected. Additional research discovered that the real first product is a six-carbon sugar. (See Figure 8.14.) • The initial reaction of the Calvin–Benson cycle fixes one CO2 into a five-carbon compound, ribulose 1,5bisphosphate (RuBP). • An intermediate six-carbon compound forms, which is unstable and breaks down to form two three-carbon molecules of 3PG. This is the compound that Calvin discovered. • The enzyme that catalyzes the fixation of carbon dioxide is ribulose bisphosphate carboxylase/oxygenase, called rubisco. • Rubisco is the most abundant protein in the world, comprising 20 percent of all the protein in every plant leaf. The Calvin–Benson cycle is made up of three processes • The Calvin–Benson cycle uses the ATP and NADPH + H+ from the light reactions to reduce CO2 to carbohydrate. • The Calvin–Benson cycle consists of three processes: • Fixation of CO2, by combination with RuBP (this is catalyzed by rubisco). • Conversion of fixed CO2 into carbohydrate (G3P) (this step uses ATP and NADPH). • Regeneration of the CO2 acceptor RuBP by ATP. • The end-product of the cycle is glyceraldehyde 3-phosphate, G3P, which is a three-carbon sugar-phosphate, also called triose phosphate. (See Figure 8.13.) • There are two fates for the G3P: • One-third ends up as starch, which is stored in the chloroplast and serves as a source of glucose. • Two-thirds is converted to the disaccharide sucrose, which is transported out of the leaf to other organs, where it is hydrolyzed into glucose and fructose to provide a source of energy. • Most of the stored energy is released by glycolysis and cellular respiration by the plant itself during growth, development, and reproduction. • Some of the carbon of glucose becomes part of amino acids, lipids, and nucleic acids. • Some of this stored energy is consumed by heterotrophs, where glycolysis and respiration release the stored energy. Refer to Figure 8.3 for an overview of the process of photosynthesis. • The products of the Calvin–Benson cycle are vitally important to Earth’s biosphere as they are the total energy yield from sunlight conversion by green plants. Photorespiration and Its Consequences • Rubisco is remarkably identical in all photosynthetic organisms. • But rubisco sometimes fixes O2 instead of CO2, lowering the overall rate of CO2 fixation and limiting plant growth. Rubisco catalyzes RuBP reaction with O2 as well as CO2 • Rubisco is a carboxylase, adding CO2 to an acceptor molecule, RuBP. It can also be an oxygenase, adding O 2 to RuBP. • These two reactions compete with each other. When RuBP and oxygen react, one of the products is a twocarbon compound, phosphoglycolate: RuBP + O2 phosphoglycolate + 3PG. • The glycolate diffuses into membrane-enclosed organelles called peroxisomes. (See Figure 8.15.) • In the peroxisomes, a series of reactions converts glycolate to glycine. • The glycine diffuses into the mitochondria, where two glycine molecules are converted into glycerate and CO2. • This pathway is called photorespiration. • It uses the ATP and NADPH produced in the light reaction. • The net effect of photorespiration is to undo what the Calvin–Benson cycle accomplished. • CO2 is released instead of being fixed into a carbohydrate. • In many plants, photorespiration reduces the amount of carbon fixed by 25 percent. • Rubisco acts as an oxygenase if the CO2 levels are very low and the oxygen levels are very high. • Oxygen levels become very high when stomata are closed to prevent water loss (when the weather is hot and dry). • When the stomata are closed during the day, CO2 continues to be consumed and O2 continues to be produced within the leaf. As the oxygen concentration increases, the oxygenase activity also increases. • The oxygenase property of rubisco may be an artifact of a time when there was little atmospheric O2. • (See Video 8.3.) C4 plants can bypass photorespiration • Many plants, called C3 plants, first fix CO2 in the three-carbon molecule 3PG, as already described. (See Figure 8.16a.) • In corn, sugarcane, and other tropical grasses, chloroplasts with abundant rubisco are in layers in the interior of the leaf. (See Figure 8.16b.) • Like C3 plants, these plants close stomata on hot, dry days, but their rate of photosynthesis neither falls off, nor does photorespiration occur. They are called C4 plants. • The C4 plants perform normal Calvin–Benson cycles, but they have an additional early reaction that fixes CO2 without losing carbon to photorespiration. • The C4 plants have two separate enzymes for CO2 fixation in different chloroplasts, in two different locations in the leaf. • One type, PEP carboxylase, is present in the mesophyll cells near the surface of the leaf. (See Figure 8.17.) • PEP carboxylase fixes CO2 to a three-carbon acceptor compound, phosphoenol pyruvate, to form a fourcarbon fixation product, oxaloacetate. • PEP carboxylase does not have oxygenase activity. It fixes CO2 even when the level of CO2 is extremely low. • The four-carbon compounds diffuse out of the mesophyll cells into the bundle sheath cells in the interior of the leaf. • The chloroplasts of these cells contain abundant rubisco. • The four-carbon compounds are decarboxylated, losing CO2 and regenerating the three-carbon acceptors, which then diffuse back to the mesophyll cells. • C3 photosynthesis appears to have begun about 3.5 billion years ago. The C4 plants appeared only about 12 million years ago. • A hundred million years ago, the concentration of CO2 was four times that of what it is now. As the CO2 levels declined, more efficient C4 plants would have had an advantage over C3 counterparts. • See Table 8.1 for a comparison of photosynthesis in C3 and C4 plants. CAM plants also use PEP carboxylase • Other plants besides C4 species use PEP carboxylase to fix and accumulate CO2 while their stomata are closed. • Such plants include succulents, such as cacti and pineapple, and also several other kinds of flowering plants. • These plants conserve water by keeping stomata closed during the daylight hours and opening them at night. • The CO2 metabolism in these plants is called the crassulacean acid metabolism, or CAM, after the family of succulents in which it was discovered. • In CAM plants, CO2 is fixed initially in the mesophyll cells to form a four-carbon compound, oxaloacetate, which is then converted to malic acid. • The fixation occurs during the night, when less water is lost through the open stomata. • The stomata close during the day, and the accumulated malic acid is shipped to the chloroplast, where decarboxylation occurs to supply CO2 for the operation of the Calvin–Benson cycle. (See Figure 8.21.) • C4 and CAM plants have evolved different solutions to the same problem. • C4 plants separate the fixation of four-carbon compounds spatially from the reactions of the Calvin–Benson cycle. • CAM plants make the same separation temporally. Metabolic Pathways in Plants • Green plants are autotrophs and can synthesize all their molecules from three simple starting materials: CO 2, H2O, and NH4. • Plants use ATP and NADPH for a number of processes besides the Calvin–Benson cycle, but ATP is also needed in organs that do not photosynthesize and also during times when the sun is not shining. • To satisfy their need for ATP, plants, like all other organisms, carry out cellular respiration. Both aerobic respiration and fermentation can occur in plants, although respiration is more common. • Cellular respiration takes place both in the dark and in the light. • Photosynthesis and respiration are closely linked by the Calvin–Benson cycle. • Some G3P from the Calvin–Benson cycle can be converted into pyruvate, the end-product of glycolysis. • Some G3P from the Calvin–Benson cycle can be converted into hexose phosphates, such as glucose 1phosphate, which can enter glycolysis. • Once carbon skeletons from the Calvin–Benson cycle enter glycolysis and the citric acid cycle, they can be used anabolically to make lipids, proteins, and other carbohydrates. (See Figure 8.18.) • Energy flows from sunlight to reduced carbon in photosynthesis to ATP in respiration. • Energy can be stored in macromolecules such as polysaccharides, lipids, and proteins. • For plants to grow, energy storage must exceed energy released. Stated another way, overall photosynthesis to fixed carbon must exceed respiration. • The capture and movement of sun energy becomes the basis for ecological food chains.