From Bohr to Quantum Mechanics Handout
advertisement
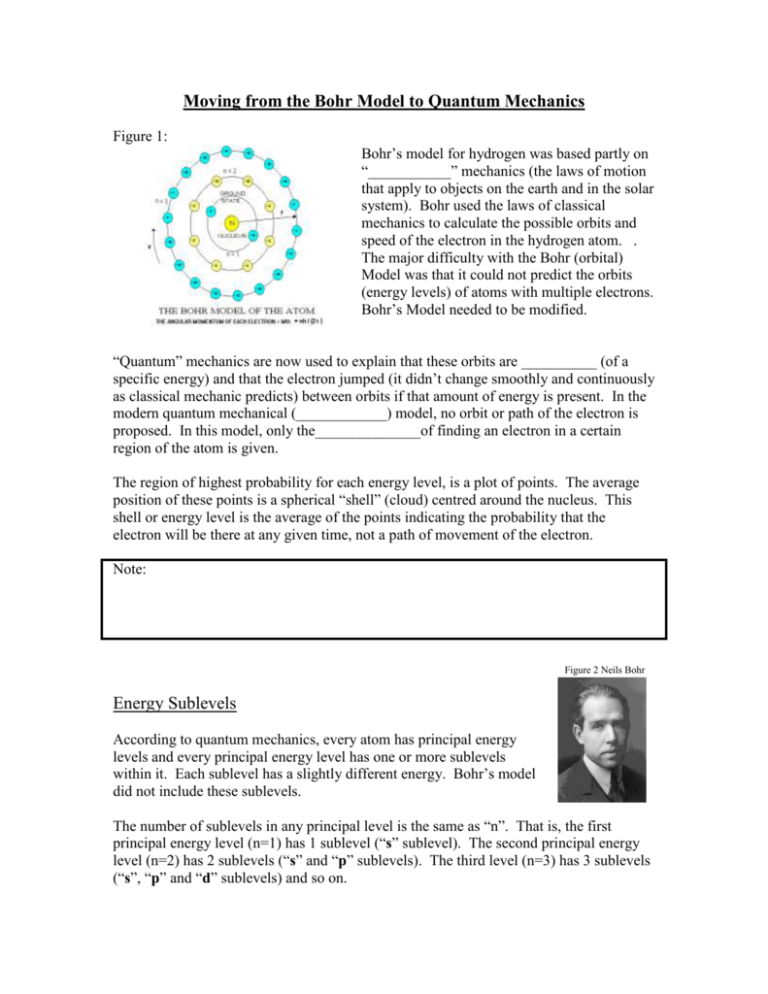
Moving from the Bohr Model to Quantum Mechanics Figure 1: Bohr’s model for hydrogen was based partly on “___________” mechanics (the laws of motion that apply to objects on the earth and in the solar system). Bohr used the laws of classical mechanics to calculate the possible orbits and speed of the electron in the hydrogen atom. . The major difficulty with the Bohr (orbital) Model was that it could not predict the orbits (energy levels) of atoms with multiple electrons. Bohr’s Model needed to be modified. “Quantum” mechanics are now used to explain that these orbits are __________ (of a specific energy) and that the electron jumped (it didn’t change smoothly and continuously as classical mechanic predicts) between orbits if that amount of energy is present. In the modern quantum mechanical (____________) model, no orbit or path of the electron is proposed. In this model, only the______________of finding an electron in a certain region of the atom is given. The region of highest probability for each energy level, is a plot of points. The average position of these points is a spherical “shell” (cloud) centred around the nucleus. This shell or energy level is the average of the points indicating the probability that the electron will be there at any given time, not a path of movement of the electron. Note: Figure 2 Neils Bohr Energy Sublevels According to quantum mechanics, every atom has principal energy levels and every principal energy level has one or more sublevels within it. Each sublevel has a slightly different energy. Bohr’s model did not include these sublevels. The number of sublevels in any principal level is the same as “n”. That is, the first principal energy level (n=1) has 1 sublevel (“s” sublevel). The second principal energy level (n=2) has 2 sublevels (“s” and “p” sublevels). The third level (n=3) has 3 sublevels (“s”, “p” and “d” sublevels) and so on. The lowest sublevel in any principal level is the “s” sublevel Ex: 1s (1st principal energy level) or______ 2s (2nd “ “ “ ) or ______ 3s (3rd “ “ “ ) or _______ The next higher sublevel is the “p” sublevel. Remember there is no “p” sublevel when n=1. For n=2 and higher (n=3, n=4 etc) “p” sublevels exist in addition to the “s” sublevels Figure 3: First Four Principal Energy Levels: Ex: 1s 2s 3s 4s 2p 3p 4p n=1 n=2 n=3 n=4 n=4 When n=3 the 3rd sublevel appears, there is a 3rd sublevel called the “d” sublevel. The “d” sublevel only appears in the energy levels of n=3 or higher n=3 n=2 Ex. 1s 2s 3s 4s 2p 3p 4p n=1 3d 4d 4f When n=4 there is a fourth sublevel, “f”. Additional sublevels, (g,h, etc) are available in higher energy levels (but won’t be discussed) Note: Orbitals Calculations from quantum mechanics have given use to better probability plots of energy levels. These plots have not only identified energy sublevels, but regions within the sublevels where electrons are likely to be found, called Orbitals (NOT ORBITS). The total number of orbitals available in an atom is predicted by: ____________ The laws of quantum mechanics allow for at most 2 electrons in each orbital. So the three “p” orbitals can have 3x2 = 6 electrons occupying them, likewise, “d” orbitals can have 5x2=10 electrons. The maximum number or electrons can be predicted by: _________ Ex. n=1 can have a total of _______________ n=1 can have a total of _______________ n=1 can have a total of _______________ n=1 can have a total of _______________ Review: Atoms contain energy levels, which contain sublevels, which themselves contain orbitals. Energy Levels = Orbitals = Total electrons = _______________ _____________ _______________ Shapes of Orbitals Figure 3: Plots of the charge clouds reveal that different shapes exist for each type of orbital. All “s” orbitals, regardless of which principal energy level they are part of (1s, 2s, 3s...), are ___________ in shape. The differences between them are that those in higher energy levels have ________________ than the lower ones. In a “p” orbital an electron has a “__________l” or “_________” shape. Evidence shows that there are three p orbitals each having the same amount of energy and the same shape and size (degenerate). How they differ is in how the orbital (dumbbell shape) is orientated – along the x-axis, y-axis or z-axis. These orbitals are given the names, px, py, pz respectively. The shapes of the “d” and “f” orbitals are more complicated and won’t be considered in chemistry 20. Questions: 1. What was the major problem with Bohr’s atomic model? 2. What information about the location of an electron is given by its principal quantum number? 3. What symbol represents the principal quantum number? 4. In the quantum mechanical model of the atom, what “new parts” were added to the principal energy levels of Bohr’s model? 5. a. How many sublevels can be present in: i. Energy level 3 ii. Energy level 6 b. How many orbitals are present in i & ii above? c. What is the maximum number of electrons that can be present in i & ii above? 6. What is an orbital? How many electrons can occupy an orbital? Taking care of those electrons - Energy Level Diagrams To help us understand what energy levels, sublevels and orbitals electrons occupy in atoms that have more than one electron (He and on) we use the energy level diagram: But first, we require some tools to use it. Note: 1. The energy of the electrons increase as we move add electrons (we move up the chart) 2. The energy levels (what n is equal to) 3. Te energy sublevels (s, p, d, f…) 4. The orbitals as o When filling the energy level diagrams, we use the following notations: 1 Electron 2 Electrons Remember an orbital can have TWO electrons MAX!! How to fill the chart: 3 Principles to follow: 1. Fill orbitals with the lowest energy first, starting at n=1 (Ground state), then move up 2. Maximum 2 electrons can occupy an orbital 3. When electrons fill orbitals of the same energy and type (i.e. 2px, 2py, 2pz) each orbital must be occupied by a single electron before they start “doubling up” We will look at four ways to write electron configurations, the first two are: 1. Orbital Notation: 2. Electron Configuration Examples: The next notation is: 3. Shortcuts: When writing out electron configurations and orbital notations for elements with large numbers of electrons, we can use a shortcut! Steps: 1. Find the last nobel gas that the element has gone past 2. Write that element in square brackets 3. Start the orbital notation/electron configuration with the next principal level and continue as normal Ex: A quick review: Element Li C Ne K Si # Electrons Electron Arrangement/Configuration Number of electrons per level Orbital Notation and Electron Configuration of Ions Recall: An ion is an atom that has a positive of negative charge and so has either lost or gained electrons. Metals (elements on the left side of the periodic table) tend to lose electrons. Nonmentals (elements on the right side of the periodic table) tend to gain electrons. This is because elements like to have the same number of electrons (become isoelectronic) as the closest nobel gas. They remain as the same element (the number of protons doesn’t change, only the number of electrons). As we will see later in this course, this is an important concept when it comes to bonding and forming compounds. Becoming isoelectronic with nobel gases is energetically favourable in elements, these elements are very stable. We can show orbital notation and electronic configuration for ions as well. Ex: The forth notation is: 4. Lewis Diagrams aka Electron dot notation In Lewis diagrams we look only at the valence electrons. Valence electrons are the outside shell s and p orbitals only, these are the most important electrons and used in bonding! Group # I II III IV V VI VII VII Li Be B C N O F Ne Na Mg Al Si P S Cl Ar Element Note: Valence electrons are the same as the group number!! Time for Practice Complete the following: Element Electrons Electron Configuration # of Electron Dot (Lewis Valence Formula) Electrons Be N Cl K Ne He I Want to learn more about quantum mechanics? Check out this website: http://www.howe.k12.ok.us/~jimaskew/cquantum.htm






