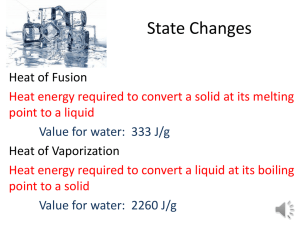
HW 12.B - Derry Area School District
advertisement

HW 12.B Name:____________________________ Physics Date:_______________ Period:_______ Section 12.2 Changes of State and Pressure In your textbook, read about changes of state on pages 323-324. Circle the letter of the choice that best completes the statement or answers the question. 1. The energy needed to melt 1 kg of a substance is called the _____. a. heat of vaporization c. heat of fusion b. condensation d. freezing point 2. The temperature at which all added thermal energy is used to change a liquid to a gas is the _____. a. boiling point c. heat of fusion b. melting point d. heat of vaporization 3. How does the amount of energy absorbed by 1 g of ice as it melts compare to the amount of energy released by 1 g of water as it freezes? a. More energy is released by the ice than is absorbed by the water. b. More energy is absorbed by the water than is released by the ice. c. The amounts of energy absorbed and released depend on the surrounding temperature. d. They are the same. 4. How does the amount of energy needed to melt 1 kg of ice at 0C compare to the amount of energy needed to change 1 kg of water to steam at 100C? The heat of fusion of water is 3.34x105 J/kg, and the heat of vaporization of water is 2.26x106 J/kg. a. It takes about two-thirds as much energy to boil water as to melt the ice. b. It takes the same amount of energy to boil the water and melt the ice. c. It takes about two-thirds as much energy to melt the ice as to boil the water. d. It takes more than six times as much energy to boil the water as to melt the ice. 1 5. As liquid changes to a gas, _____. a. both its kinetic and potential energy increase c. only its potential energy increases b. only its kinetic energy increases d. neither its kinetic nor potential energy increases Short Answer 6. Why does the temperature of H2O not rise as ice changes to water and water changes to steam? 7. Why would you get a worse burn from contact with 1 g of steam at 100C than from contact with 1 g of water at the same temperature? 8. You know that you can measure the transfer of heat. Can you measure cold? Explain your answer. 2 Properties of Fluids In your textbook, read about pressure on page 342. For each statement below, write true or rewrite the italicized part to make the statement true. 1. ___________________________________ Fluids flow and have no definite volume. 2. ___________________________________The SI unit of pressure is the pascal, Pa. 3. ___________________________________Pressure is inversely proportional to force. 4. ___________________________________Fluids have no definite shape of their own. 5. ___________________________________The forces that hold molecules of the floor together cause the floor to exert an upward pressure on your feet. 6. ___________________________________As you move to higher altitudes on Earth, atmospheric pressure increases. 7. ___________________________________The typical pressure at the center of the Sun is greater than the typical pressure at the center of Earth. 8. ___________________________________1 Pa is equivalent to 1 N/m2. 9. ___________________________________Standard atmospheric pressure is equal to 101.3 kPa. Short Answer 10. How does a gas exert pressure? Why does heating a gas increase the pressure? 3 Problems 11. A car tire makes contact with the ground on a rectangular area of 12 cm by 18 cm. If the car’s mass is 925 kg, what pressure does the car exert on the ground as it rests on all four tires? 12. A sheet of paper 3.0 cm by 4.0 cm is lying on the desk. If the atmospheric pressure is 1.013 x 105 Pa, what is the total force exerted on the paper by the atmosphere? 4