Matter Test Review Packet
advertisement
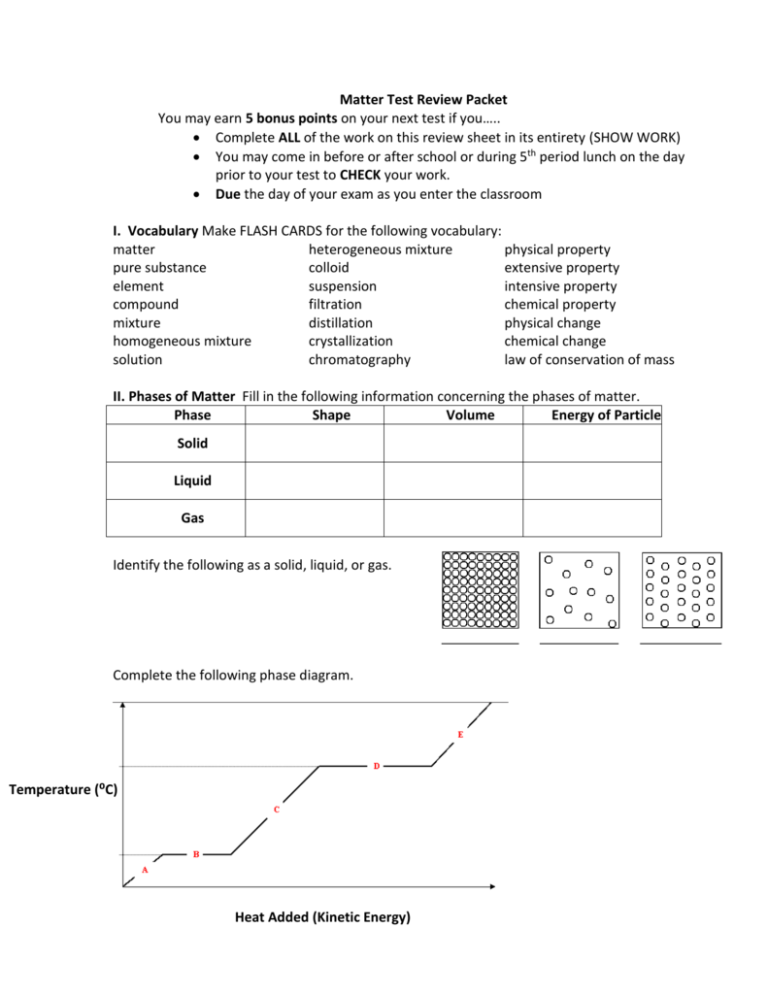
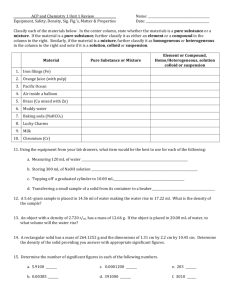
Matter Test Review Packet You may earn 5 bonus points on your next test if you….. Complete ALL of the work on this review sheet in its entirety (SHOW WORK) You may come in before or after school or during 5th period lunch on the day prior to your test to CHECK your work. Due the day of your exam as you enter the classroom I. Vocabulary Make FLASH CARDS for the following vocabulary: matter heterogeneous mixture physical property pure substance colloid extensive property element suspension intensive property compound filtration chemical property mixture distillation physical change homogeneous mixture crystallization chemical change solution chromatography law of conservation of mass II. Phases of Matter Fill in the following information concerning the phases of matter. Phase Shape Volume Energy of Particles Solid Liquid Gas Identify the following as a solid, liquid, or gas. Complete the following phase diagram. Temperature (⁰C) Heat Added (Kinetic Energy) III. Properties/Changes of Matter 1. List 10 different kinds of physical properties. 2. List 10 different kinds of chemical properties. 3. In your own words, what is the difference between a property and change? 4. To the left of each sentence, identify each of the following as a physical(P) or a chemical(C) change. a. You leave your bicycle out in the rain and it rusts. b. A sugar cube dissolves in water. c. Sand is mixed with water. d. You are cleaning your bathroom and you accidentally mix bleach and ammonia, which produces a toxic gas, which makes you pass out. e. Burning coal for a barbecue. f. Chewing up a bite of hamburger. g. You take a shower and your wet hair begins to dry. h. Trimming a plant because it has grown too high. i. Mashing up potatoes to make mashed potatoes. j. Hydrogen peroxide is poured on some liver the liver begins to break down. 5. Identify each statement as being true or false to the left of the sentence. a. A change in size or shape is a physical change. b. A chemical change means a new substance with new properties was formed c. An example of a chemical change is when water freezes. d. When platinum is heated, then cooled to its original state, we say this is a physical change. e. When milk turns sour, this is a physical change because a change in odor does not indicate a chemical change. f. When magnesium is burned, ash forms. We say this is a physical change because the magnesium looks different. g. When citric acid and baking soda mix, carbon dioxide is produced and the temperature decreases. This must be a chemical change. IV. Conservation of Mass 1. A 28g sample of nitrogen gas combines completely with 6g of hydrogen gas to form ammonia. What is the mass of ammonia formed? Matter Test Study Guide (Your HELPMATE!!!) 1. 2. 3. 4. 5. What is Matter? What are the phases of matter? Describe the energy of matter particles in each phase. Be able to identify each part of a phase diagram. Matter can be classified as a pure substance and a mixture. What is the difference between these classifications? 6. What is a pure substance substance? 7. Pure substances can be classified as two types of matter. What are they? Be able to describe each type and give an example of each. 8. What is a mixture? 9. Mixtures can be classified as two types of matter. What are they? Be able to describe each type and give an example of each. 10. What is the difference between a solution, colloid, and suspension? Be able to compare their particle sizes. 11. What are the four methods of separation that we discussed? Be able to describe and/or identify each. 12. What is a property? 13. Explain the difference between a physical and chemical property. 14. What is an extensive property? 15. What is an intensive property? 16. What is a change? 17. Explain the difference between a physical and chemical change. BE SURE TO CONTINUE TO STUDY THE METRIC SYSTEM, THE FACTOR LABEL METHOD AND DENSITY. YOU WILL SEE IT AGAIN!!!!!!!!!!!!!!!!!!