Formal Lab Report Template
advertisement

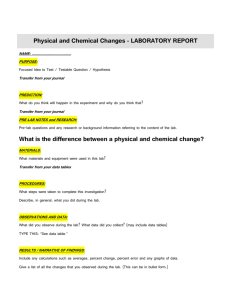
Formal Lab Reports Mr. Smith’s Honors Chemistry Honors chemistry students will write at least one formal lab report per semester, as assigned by Mr. Smith. The report will contain all relevant information and your analysis regarding a particular lab. Your formal lab report should contain each of the sections outlined below. These sections should be clearly marked with section headings, and should contain the specified information. Unless you submit a written request to me, which I subsequently approve prior to the report’s due date, the report must be typed (word-processed) and single-spaced. There is no length requirement on formal lab reports, but it will probably be around 4-6 pages. Title Page: Title, Date, and Your Name and Names of Lab Partners On a separate cover page, you should list the title of the report, the date you conducted the experiment, the date you submitted the report, your name and the names of those who worked in your group. The title can be fairly long and should describe the principles behind the lab. For example, a report that examines the principles of the law of conservation of mass should be titled something like: “A Report Exploring the Law of Conservation of Mass.” Introduction (1 page, consisting of 3-4 paragraphs) The introduction presents the theory or theories behind the laboratory experiment. It should be an overview of the concept that the lab illustrates. The introduction should follow a succinct train of thought. The first paragraph of the introduction should outline the general chemistry concept. The second paragraph should be more specific, focusing on a subset of the concept relevant to the particular study. The third paragraph should be even more specific, discussing how the concept relates the experiment. For example, you may do a lab that analyzes the densities of various solid compounds. The first paragraph of your introduction should explain density. The second paragraph should explain, more specifically, the density of solids. Your third and final paragraph should discuss the density of the particular compounds (or class of compounds) included in the laboratory experiment. Purpose or Hypothesis (1-2 sentences) This section comes directly from your pre-lab. After completing the lab, you may find that your hypothesis is incorrect. That is fine. Many chemical discoveries happen when experiments yield unexpected results. If the hypotheses you put forth in your pre-lab notes is incorrect, do not state that “my hypothesis, as stated in my pre-lab notes (which said etc., etc., etc.) was incorrect and it is now etc., etc., etc.” Instead, leave the analysis of your original hypothesis and its confirmation or refutation to the “Discussion” section of the report (described below.) Materials (1/2 page list) The materials you actually used for the lab should be presented as a list and should include all glassware, instruments, and chemicals. Be sure to designate the sizes Version 1.0 Page 1 Formal Lab Reports Mr. Smith’s Honors Chemistry of the glassware, types of instrument, and quantities of chemicals and to use their proper names. The final list of materials will usually differ from the list in your pre-lab, depending on what equipment and chemicals were available at the time you did your experiments. Be sure that your “Materials” section reflects the chemicals, quantifies, instruments and glassware actually used! Procedure (1-2 pages - paragraphs, depending on the lab) The “Procedure” section should be a very specific list of steps written in full sentences. Like the “Materials” section, your “Procedure” section should include changes made to the pre-lab and should reflect what you actually did during the lab period. If you added steps, include them. If you altered steps, change them. Be specific. Include specific time-intervals and temperatures. For example, you should not write, “Stir the compound while heating it.” Instead you should write, “Stir the compound with a glass stir rod for 5 minutes while heating it in a water bath at 100C.” If you repeat steps for more than one compound, you may list a numbered step reflecting this repetition. For example, you may write, “Repeat steps 4-9 for sodium chloride, aluminum sulfite, and barium nitrate.” Data (1-2 pages, including tables and recorded observations) Data are observations you collect during the laboratory experiment. You should summarize the data in a manner that is easy to view. I suggest making a data table, especially for quantitative (numerical) data. Graphs can be a good way to show the relationship between many numbers. Make sure that every number has a unit associated with it. Qualitative (descriptive) data also belongs in this section, either in a table or written in paragraph form. Do not analyze your data in this section. Results (1-2 pages, including calculations, tables and graphs) In this section, examine (discuss) but do not analyze your quantitative data. Show all of your calculations, including error calculations. After completing calculations, present the numbers in a clear format. It is usually easiest to organize your quantitative results in a table. This will make it easier for you to analyze the results in your discussion section. Discussion (1/2-1 page) After presenting your data and doing calculations, you must analyze your results. This section is an interpretation of your data, both quantitative and qualitative. For quantities, explain what the numbers mean. You should also discuss your qualitative data. You must interpret the meaning behind your observations. For example, if you stated in your “Data” section that the reaction produced heat, you should state in your “Discussion” section that the reaction was exothermic. You are explaining the meaning behind your data and results. Discuss whether your hypothesis was correct or not and if you achieved the purpose of the lab. After explaining your data and results, explain sources of possible error. Include inherent error of equipment, as well as human error. Version 1.0 Page 2 Formal Lab Reports Mr. Smith’s Honors Chemistry The discussion is the most important section of the report, since it reveals your comprehension of the topic and its relationship to the experiment. Be sure to reveal your understanding! Conclusion (1 paragraph) In conclusion, you should summarize the salient aspects of your experiment. Discuss, technically, what you learned from the lab and how the experiment helped in your understanding of the topic. If your lab did not turn out well, explain what you think went wrong. If you had to do the lab again, what would you do differently? How could you eliminate some of your error? This section does NOT replace your reflection that will be due at the same time your lab report is due. Grading Rubric The objectives of all lab reports are two-fold: firstly, to provide enough detailed and descriptive data for a reader to duplicate your experiment and, secondly, to discuss the results obtained in a lab and either support or refute your original hypothesis. Irrespective of the rubric guidelines, below, a lab report must do these two things! Lab Reports will be graded on a “Check Plus, Check and Check Minus” (A, B, C) scale with half a grade adjustments up or down, based upon grammar, structure, spelling and organization. A perfect lab report (“100”) will include all of the sections listed above, thoroughly developed, be technically correct and be essentially free of spelling and grammar errors. A “Check Plus” lab report will include all of the sections listed above but may contain minor technical errors, will be less well developed and may contain minor spelling or grammar errors. A “Check” lab report will include all of the sections listed above but may fail to develop a major section of the report and may contain several spelling or grammar errors. A “Check Minus” lab report will (essentially) omit one or more sections of the report and may contain numerous spelling or grammar errors. (Note: This document was derived largely from a presentation by Ms. Joslyn Pretz of Glenelg High School Honors Chemistry, Glenelg, Maryland.) Version 1.0 Page 3