Gummy Bear Osmosis
advertisement

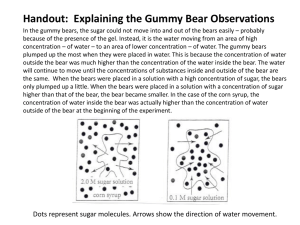
Gummy Bear Osmosis Name ___________________________________ Date ______ Period ____________ Seat ______ Diffusion is defined as the movement of molecules from an area of high concentration to an area of low concentration. The diffusion of water molecules through a selectively permeable membrane is known as osmosis. Movement through membranes is called transport. Diffusion and osmosis are passive forms of transport; this means that they do not need energy to move from areas of high concentration to areas of low concentration. Active transport requires energy to transport molecules from low concentration to high concentration. Question: How will soaking Gummy Bear candies in distilled water affect their size? Prediction (explain your prediction based on the background information): Materials: (Groups of 3) Plastic cup (1) Gummy Bears (3 of different colors) Metric ruler Distilled water Electronic balance Calculator Figure 1 Procedure: Day 1 1. Use a pen to label your cup with your names & class period. 2. Obtain three different colored Gummy Bears. 3. Use a ruler to measure the length, width, and height of each Gummy Bear in centimeters, ±0.1cm (see Figure 1). Record these measurements in your data table on page 2. 4. Calculate the volume (in cm3) of each Gummy Bear and record these values in your data table. 5. Use an electronic balance to find the mass (in grams) of each of your Gummy Bears. Record these masses in your data table. 6. Use a marker to divide the bottom of your cup into three equal zones. Place a letter in each zone that represents the colors of your Gummy Bears (see Figure 2) 7. Fill your cup with distilled water (NOT tap water) 8. Lay each Gummy Bear in the cup within its corresponding zone. 9. Leave the Gummy Bears in the water overnight. length Figure 2 R G W R = red G = green W = white 1 height width O = orange Y = yellow B = blue Gummy Bear Osmosis Name ___________________________________ Date ______ Period ____________ Seat ______ Part 2: The next day 1. After the Gummy Bears have been in distilled water overnight, GENTLY take one Gummy Bear out of the water and pat it dry. 2. Use your ruler to measure the length, width, and height (in cm) of the Gummy Bear. Record these values in your data table under the correct Gummy Bear color. 3. Calculate the volume (in cm3) of the Gummy Bear and record this value in your data table. 4. Use an electronic balance to find the mass (in grams) of your Gummy Bear. Record this value in your data table. 5. Repeat steps 1-4 with the other two Gummy Bears. Data Dimensions Gummy Bear #1 (Color:_______________) Initial Final Before After Soaking Soaking (Day 1) (Day 2) Gummy Bear #2 Gummy Bear #3 (Color:______________) (Color:_______________) Initial Final Initial Final Before After Before After Soaking Soaking Soaking Soaking (Day 1) (Day 2) (Day 1) (Day 2) Length (cm) Width (cm) Height (cm) Volume (cm3) LxWxH= Mass (grams) Questions and Analysis: 1. What type of solution (hypertonic, isotonic, or hypotonic) did you place the Gummy Bear in, and what happened to the Gummy Bear as a result of being placed in the solution (Look at your Day 1 vs. Day 2 results)? Explain the direction of water movement. _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ 2. What do you think will happen if the Gummy Bears were placed in salt water on day 2 and left in the salt solution until day 3? _________________________________________________________________________________ _________________________________________________________________________________ ________________________________________________________________________________ 2 Gummy Bear Osmosis Name ___________________________________ Date ______ Period ____________ Seat ______ 3. Before soaking the Gummy Bears in distilled water, the distilled water tested negative for glucose. Now perform a glucose test using Benedicts solution on the water that the bear was soaking in. a. What were the results? _____________________________________________________ b. Show me the results for credit. __________________ (my initials) c. Explain why glucose (is/isn’t) found in the soaking water: _______________________ ________________________________________________________________________ Calculate the percent change in the size of the candy: 4. % Change in Volume Gummy Bear #1 ( _____________ After soaking volume Gummy Bear #2 Gummy Bear #3 ˗ _____________ ) Before soaking volume ( _____________ ˗ _____________ ) After soaking volume Before soaking volume ( _____________ ˗ _____________ ) After soaking volume Before soaking volume _______________ x 100 = _______% Before soaking volume _______________ x 100 = _______% Before soaking volume _______________ x 100 = _______% Before soaking volume Average % Change in Volume ( _______________% + ________________% + _________________% ) / 3 = _______________% % change of bear #1 5. % change of bear #2 % change of bear #3 % Change in Mass Gummy Bear #1 ( _____________ After soaking mass Gummy Bear #2 ( _____________ After soaking mass Gummy Bear #3 ( _____________ After soaking mass ˗ _____________ ) Before soaking mass ˗ _____________ ) Before soaking mass ˗ _____________ ) Before soaking mass _______________ x 100 = _______% Before soaking mass _______________ x 100 = _______% Before soaking mass _______________ x 100 = _______% Before soaking mass Average % Change in Mass ( ________________% + ________________% + ________________% ) / 3 = _______________% % change of bear #1 % change of bear #2 % change of bear #3 3 Gummy Bear Osmosis Name ___________________________________ Date ______ Period ____________ Seat ______ 6. Graph the average percent changes on a bar graph. Remember to title and label both axes. 7. Conclusion: Write a conclusion for this controlled experiment. In your conclusion, be sure to: Answer the experimental question. Include supporting data from your data table. Explain how this data supports your conclusion. Provide a scientific explanation for the trend in the data. Question: How does soaking Gummy Bears in distilled water affect the size of the candy? 4 Gummy Bear Osmosis Name ___________________________________ Date ______ Period ____________ Seat ______ Plan a controlled experiment to answer the question below. You may use any materials and equipment in your procedure. Be sure your procedure includes: • Logical steps to do the experiment • Two controlled (kept the same) variables • One manipulated (independent) variable • One responding (dependent) variable • How often measurements should be taken and recorded Question: How is the volume of the Gummy Bears affected by the percent salt water solution they are placed in? 5 Gummy Bear Osmosis Name ___________________________________ Date ______ Period ____________ Seat ______ Conclusion Scoring Rubric Attributes Conclusive statement: Supporting data for Percent change in Volume: Supporting data for Percent change in Mass: Explanatory language: Scientific explanation: Total Attributes and Score Points Attribute Name Credit Description / Evidence of Attribute Controlled Variables (need at least two) Manipulated Variable Responding Variable Record Measurements Trials are Repeated Extra Validity Measure Logical Steps Total Attributes Score Points 6