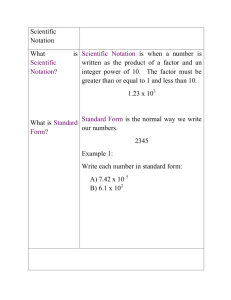
Section 3 Worksheet / Review
advertisement
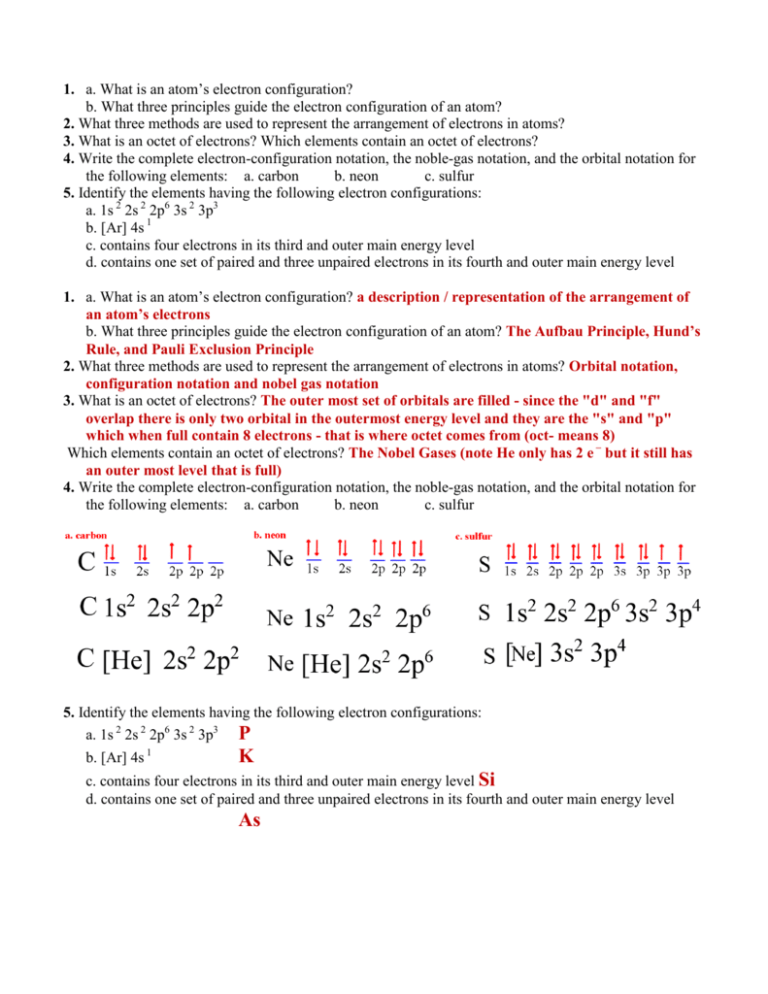
1. a. What is an atom’s electron configuration? b. What three principles guide the electron configuration of an atom? 2. What three methods are used to represent the arrangement of electrons in atoms? 3. What is an octet of electrons? Which elements contain an octet of electrons? 4. Write the complete electron-configuration notation, the noble-gas notation, and the orbital notation for the following elements: a. carbon b. neon c. sulfur 5. Identify the elements having the following electron configurations: a. 1s 2 2s 2 2p6 3s 2 3p3 b. [Ar] 4s 1 c. contains four electrons in its third and outer main energy level d. contains one set of paired and three unpaired electrons in its fourth and outer main energy level 1. a. What is an atom’s electron configuration? a description / representation of the arrangement of an atom’s electrons b. What three principles guide the electron configuration of an atom? The Aufbau Principle, Hund’s Rule, and Pauli Exclusion Principle 2. What three methods are used to represent the arrangement of electrons in atoms? Orbital notation, configuration notation and nobel gas notation 3. What is an octet of electrons? The outer most set of orbitals are filled - since the "d" and "f" overlap there is only two orbital in the outermost energy level and they are the "s" and "p" which when full contain 8 electrons - that is where octet comes from (oct- means 8) Which elements contain an octet of electrons? The Nobel Gases (note He only has 2 e – but it still has an outer most level that is full) 4. Write the complete electron-configuration notation, the noble-gas notation, and the orbital notation for the following elements: a. carbon b. neon c. sulfur 5. Identify the elements having the following electron configurations: a. 1s 2 2s 2 2p6 3s 2 3p3 P b. [Ar] 4s 1 K c. contains four electrons in its third and outer main energy level Si d. contains one set of paired and three unpaired electrons in its fourth and outer main energy level As