Sample Rate Law and Activation Energy
advertisement

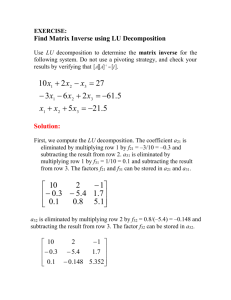
Sample Rate Law and Activation Energy (Ea) Calculations 2013, Sharmaine S. Cady East Stroudsburg University The following set of rate data was collected for the reaction between I- and H2O2. Table 1. Reagent Volumes for Kinetic Trials at 21.0 °C Solution A Kinetic Trial Deionized water, mL 1 2 3 4 5 6 7 8 4.0000 3.0000 2.0000 1.0000 2.0000 0.0000 5.0000 2.0000 Buffer, 0.500 M HAc and 0.4998 M NaAc, mL 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 Solution B 0.3001 M KI, mL 0.0201 M Na2S2O3, mL Starch 0.0998 M H2O2, mL 1.0000 2.0000 3.0000 4.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 1.0000 5 drops 5 drops 5 drops 5 drops 5 drops 5 drops 5 drops 5 drops 3.0000 3.0000 3.0000 3.0000 5.0000 7.0000 2.0000 4.0000 Time, s 65.00 31.00 21.00 15.00 37.00 27.00 96.00 49.00 The rate of the reaction for each trial is calculated by taking the [I2] for each trial and dividing by the time in seconds. 1 How to find [I2] The number of moles of I2 present at the start of the reaction is 0. As the reaction proceeds, the redox reaction produces I2. The number of moles I2 present at the time the blue-black color appears can be determined from the stoichiometry between I2 and the amount of S2O32- at the start of the reaction. The equation for the number moles of I2 present when the blue black color appears is given on the following page. stoichiometric ratio from reaction equation calculated Na2S2O3 M volume Na2S2O3 micropipetted Rate laws use concentrations in units of molarity (M). To calculate the [I2] in units of molarity, the number of moles of I2 formed must be divided by the total volume of the reaction mixture in units of liters (L). Summing the volumes of all reagents gives 10.0000 mL as the total reaction volume: The two equations can be combined to determine the [I2]: Since the volume of NaS2O3 micropipetted is the same for all trials, [I2] is the same for all trials. The molarity of the thiosulfate solution has 3 significant digits, the volume pipetted has 5, and the total reaction volume has 6. Therefore, the value of [I2] is rounded off to the lower number, which is 3 significant digits. 2 How to find rate (M/s) [I2] is divided by the time for each trial. The data from Trial 1 is used in the following calculation. Since all the times have 4 significant digits and [I2] has 3, the rates are rounded off to the lower number, which is 3 significant digits. Note the units on the rate. This calculation is repeated for the next seven trials and their measured time values. How to find the reaction order with respect to [I-] To determine how the rate depends on [I-], select the trials where the volume of H2O2 is held constant. This gives a constant [H2O2], which means the rate only depends on [I-]. For this experiment, trials 1-4 are used because the volume of H2O2 remains constant at 3.0000 mL. Determine the initial concentration of [I-]0 from the volume of KI micropipetted, reagent concentration, and the total volume of the reaction mixture. This is a dilution calculation and the following equation may be used: For this experiment, Mc = original concentration KI (0.3001 M), Vc= volume micropipetted, MD = [KI]0, and VD = reaction solution volume (10.0000 mL). Substituting the values for trial 1 into the above equation and rearranging to solve for MD ([KI]0) yields: ( )( ) ( ) ( )( ) ( ) This is also [I-]0 since, given the formula stoichiometry, there is 1 mole of I- for every mole of KI. This calculation is repeated for trials 2-4 where the Vc volume is the only variable that changes. The log values of the rate and [I-]0 for each trial are computed. To express the log values with the correct number of significant digits, count the number of significant digits in the [I-]0, and express the log value with the same number of decimal places. The log value for the rate is expressed in a similar manner. Log values have no units. 3 Table 2. Log Values for [I-]0 and Rate [I-]0, M 0.03001 0.06002 0.09003 0.1200 Trial 1 2 3 4 Log [I-]0 -1.5227 -1.2217 -1.0456 -0.9208 Rate, M/s 1.55 x 10-5 3.26 x 10-5 4.81 x 10-5 6.73 x 10-5 Log Rate -4.810 -4.487 -4.318 -4.172 Note the [I-]0 values have 4 significant digits and the log values have 4 decimal places. The rate has 3 significant digits and the log values have 3 decimal places. The log rate vs. log [I-]0 is plotted to obtain a linear plot with given slope. The slope rounded off to the nearest whole integer is the reaction order with respect to [I-]. Log Rate vs. Log [I-]0 -1.6000 -1.4000 -1.2000 -1.0000 -0.8000 -0.6000 -0.4000 -0.2000 -4.100 0.0000 -4.200 y = 1.048x - 3.2142 R² = 0.9993 -4.300 log rate -4.400 -4.500 -4.600 -4.700 -4.800 log [I-]0 4 -4.900 How to find the reaction order with respect to [H2O2] To determine how the rate depends on [H2O2], select the trials where the volume of I- is held constant. This gives a constant [I-], which means the rate only depends on [H2O2]. For this experiment, trials 1 and 5-7 are used because the volume of KI remains constant at 1.0000 mL. Determine the initial concentration of [H2O2]0 from the volume of H2O2 micropipetted, reagent concentration, and the total volume of the reaction mixture. This is a dilution calculation and the following equation may be used: For this experiment, Mc = original concentration KI (0.0998 M), Vc= volume micropipetted, MD = [H2O2]0, and VD = reaction solution volume (10.0000 mL). Substituting the values for trial 1 into the above equation and rearranging to solve for MD ([KI]0) yields: ( )( ) ( ) ( )( ) ( ) This calculation is repeated for trials 5-7 where the Vc volume is the only variable that changes. The log values of the rate and [H2O2]0 for each trial are computed. Table 3. Log Values for [H2O2]0 and Rate Trial 1 2 3 4 [H2O2]0, M 0.0299 0.0499 0.0699 0.0200 Rate, M/s 1.55 x 10-5 2.73 x 10-5 3.74 x 10-5 1.05 x 10-5 Log [H2O2]0 -1.524 -1.302 -1.156 -1.699 Log Rate -4.810 -4.564 -4.427 -4.979 Note the [H2O2]0 values have 3 significant digits and the log values have 3 decimal places. The rate has 3 significant digits and the log values have 3 decimal places. 5 The log rate vs. log [H2O2]0 is plotted to obtain a linear plot with given slope. The slope rounded off to the nearest whole integer is the reaction order with respect to [H2O2]. Log Rate vs. Log [H2O2]0 -1.800 -1.600 -1.400 -1.200 -1.000 -0.800 -0.600 -0.400 -4.300 -0.200 0.000 -4.400 y = 1.0255x - 3.2399 R² = 0.999 -4.500 log rate -4.600 -4.700 -4.800 -4.900 -5.000 log [H2O2]0 -5.100 How to find k’ Since the slopes rounded off to the nearest whole integer are both 1 for the two reactants, the rate law becomes The reaction is first order with respect to [I-] and [H2O2] and second order overall. The computed rates for trials 1-8 and the computed initial concentrations of I- and H2O2 are substituted into the above equation. These are the same values used to obtain the log values for the plots. The equation is then solved for k’. 6 Table 4. Calculated k’ Values Trial 1 2 3 4 5 6 7 8 [I-]0 , M 0.03001 0.06002 0.09003 0.1200 0.03001 0.03001 0.03001 0.03001 Rate, M/s 1.55E-05 3.24E-05 4.79E-05 6.70E-05 2.72E-05 3.72E-05 1.05E-05 2.05E-05 [H2O2]0, M 0.0299 0.0299 0.0299 0.0299 0.0499 0.0699 0.0200 0.0399 AVERAGE k’ STD DEV k’, M-1s-1 0.0172 0.0180 0.0178 0.0186 0.0181 0.0178 0.0175 0.0171 0.0178 0.0005 Substitution of the values from Trial 1 yields the following for k’: ⁄ ( ( )( )( ) ) The rate and [H2O2]0 both have 3 significant figures and [I-]0 has 4. The answer is rounded off to the lower number of significant digits, which is 3. This calculation is repeated for the next seven trials and their measured rates and concentrations. Using Excel to compute the average and standard deviation Enter the eight values for k’ into the cells for the first column. Click in the cell below the last entry. Click on Formulas on the menu ribbon and then Insert Function. Select AVERAGE in the function box. Then OK. Make sure the dialog box shows the correct range for the four entered values. Click OK. Click the cell below the average value. Click on Insert Function. Select STDEV in the function box. Make sure the dialog box shows the correct range of entered values. Click OK. The standard deviation is rounded off to 1 significant figure. 7 How to find Ea The rate and k’ are calculated for the two kinetic runs performed at 0 °C and 30 °C. The calculations are the same as those used for Trial 4 at room temperature. The reciprocal of the temperatures in Kelvin units are calculated. The A graph of ln k’ vs. 1/T is plotted. The average k’ from the room temperature trials is used for plotting purposes. Table 5. Rate and Temperature Data T, K (°C + 273.15) 273.2 294.2 309.2 time, s 75.00 15.00 4.00 k’, M-1s-1 3.73 x 10-3 1.78 x 10-2 6.99 x 10-2 Rate, M/s 1.34 x 10-5 -2.51 x 10 1/T 3.661 x 10-3 3.399 x 10-3 3.245 x 10-3 ln k’ -5.592 -4.029 -2.661 Ea is calculated by sustituting the slope into the following equation: ( )( ) The k’ has 3 significant digits and T has 4; therefore the slope has 3 significant digits. The red numbers are not significant but are retained until the desired answer is obtained. Ea is rounded off to 3 significant digits and expressed in units of kJ/mol. Note that the slope is negative and yields a positive value for Ea. 8 ln k' vs. 1/T 0.000 3.200E-03 3.250E-03 3.300E-03 3.350E-03 3.400E-03 3.450E-03 3.500E-03 3.550E-03 3.600E-03 3.650E-03 3.700E-03 -1.000 y = -6926.6x + 19.699 R² = 0.988 ln k' -2.000 -3.000 -4.000 -5.000 -6.000 1/T, K-1 9