Understanding Chemical Formulas
advertisement

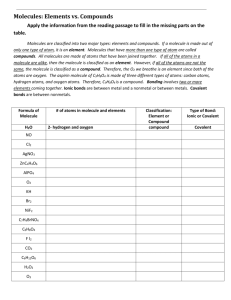
Grade Level/Course: Grade 8 / Physical Science Lesson/Unit Plan Name: Understanding Chemical Formulas Rationale/Lesson Abstract: This lesson provides practice for understanding chemical formulas. Students will identify elements in compounds using symbols on the Periodic Table. They will also understand the use of subscripts and coefficients in chemical formulas and interpret diagrams of molecules. This lesson also prepares students for understanding reactants and products, and balancing equations for Conservation of Matter. Timeframe: 1 class period Standard(s): Structure of Matter 3. Each of the more than 100 elements of matter has distinct properties and a distinct atomic structure. All forms of matter are composed of one or more of the elements. As a basis for understanding this concept: b. Students know that compounds are formed by combining two or more different elements and that compounds have properties that are different from their constituent elements. f. Students know how to use the periodic table to identify elements in simple compounds. Reactions 5. Chemical reactions are processes in which atoms are rearranged into different combinations of molecules. As a basis for understanding this concept: a. Students know reactant atoms and molecules interact to form products with different chemical properties. b. Students know the idea of atoms explains the conservation of matter: In chemical reactions the number of atoms stays the same no matter how they are arranged, so their total mass stays the same. Instructional Resources/Materials: • Students need access to a Periodic Table (one version is provided below) • Assessment handout (provided below) Page 1 of 4 MCC@WCCUSD 1/9/14 Activity/Lesson: Compounds are elements that are chemically combined and bonded together. Chemical formulas are used to describe compounds using symbols from the Periodic Table. Common examples include: • Water: H2O • Carbon dioxide: CO2 • Salt: NaCl • Glucose: C6H12O6 Periodic Table Symbols The elements present in the compound can be identified using a Periodic Table. Students should remember that some symbols are a single upper case letter, like carbon (C) or oxygen (O) while others, like Cobalt (Co) or Chlorine (Cl), have an upper case followed by a lower case letter. The element Chlorine (Cl) can be tricky, because it can be confused with Carbon (C) and Iodine (I). Subscripts The subscript is the smaller number to the lower right of each symbol. The subscripts show the number of atoms in each molecule of the compound. Where there is no number, one atom is implied. For example, in each molecule of ferric chloride: FeCl3, there is one atom of iron (Fe) and three atoms of chlorine (Cl). Parentheses Parentheses are used for groups that appear together multiple times in a molecule. For example, in calcium phosphate: Ca3(PO4)2 , there are two groups of phosphate (PO4) in each molecule. Coefficients A coefficient shows the number of whole molecules in a formula. In a chemical equation, it shows the ratio of molecules present in the reaction. Similar to a coefficient in math, it multiplies all that comes after it. Again, where there is no coefficient, a ‘1’ is implied. For example, 4H2O is 4 separate molecules of water: H-­‐O-­‐H H-­‐O-­‐H H-­‐O-­‐H H-­‐O-­‐H Assessment: Handout for practice below. Page 2 of 4 MCC@WCCUSD 1/9/14 Example: The chemical formula for carbon dioxide is CO2. Carbon dioxide is a compound, because it is made of multiple elements: carbon, oxygen. • Use the symbols on the periodic table to find which elements are present in a compound. Each molecule of carbon dioxide (CO2) has one atom of carbon and two atoms of oxygen. • The subscript tells you how many atoms of that element are present in each molecule. • The coefficient tells you the number of molecules present. • So a diagram for 3 CO2 might look like this: Understanding+Chemical+Formulas+ Answer the following. You will need a Periodic Table. 1. How many different elements are in boric acid: H3BO3? ________ 2. Which elements are in ferric chloride: FeCl3? ________________________________________ 3. Which elements are in sodium silicate: Na2SiO3? _____________________________________ 4. Which elements are in acetaminophen: C8H9NO2? ____________________________________ 5. How many boron atoms are in each molecule of boric acid: H3BO3? ________ 6. What does the ‘5’ mean in vanadium pentoxide: V2O5? ________________________________ 7. How many atoms, in total, are in each molecule of Vitamin C: C6H8O6? ________ 8. How many atoms of oxygen are in each molecule of magnesium phosphate: Mg (PO ) ? ______ 3 4 2 9. How many whole molecules are in 3 (NH ) SO ? ________ 4 2 4 10. Which of these represents 2 H O ? 2 2 a. H O O H H b. H O O H c. H H H O O O O 11. Which of these could be a model of sodium silicate, Na2SiO3? O a. O Si O O a Na Na b. a N O i S O c. Na Na SiO SiO SiO H H H d. O H H O O Na Si O d. Na Si O 12. Sketch a diagram of 3 NH3 : Page 3 of 4 O MCC@WCCUSD 1/9/14 O O H Page 4 of 4 MCC@WCCUSD 1/9/14