Chem 230 Lecture 12 Page- 1 -
advertisement

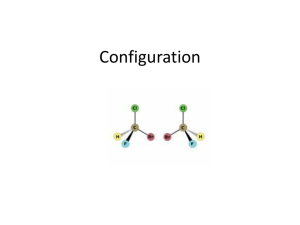
Chem 230 Lecture 12 Page- 1 - 1. Last time I left you with the following important concepts. - Other than being able to recognize stereoisomers as enantiomers or diastereomers, the most important concept is the following: Enantiomers have identical properties whereas diastereomers have different properties. There are no exceptions. i. Instructor sets up a demonstration with student volunteers. - Chiral molecules have non identical mirror images. Chirality is a spatial feature that an object possesses if it has a non identical mirror image. i. Is there a symmetry operation or a lack there of that allows us to recognize an objector a molecule as achiral? - Stereogenic atoms give rise to chirality but they are not necessary or sufficient to produce chirality. We have seen previously that they are not necessary. i. To demonstrate that they are not sufficient to produce chirality we need more than one stereogenic atom in the molecule. 2. The ubiquity of the fact that enantiomers have the same properties will amaze you. - Think about baseball or cricket. i. Statistics with all (left handed bowlers or pitchers facing right handed batsmen or hitters) will produce the same results as statistics with all (right handed bowlers or pitchers facing left handed batsmen or hitters). ii. The numbers should be the same, only reversed. iii. That is one case will give a ratio of 0.751 foul balls to the right over foul balls to the left. The other case will give 0.751 foul balls to the left over foul balls to the right. iv. The numbers with be different in general with no obvious relationship when either situation above is compared to the case with both the pitcher and batter left handed, on both right handed. v. The latter pair would be diastereomers, or diastereomeric. 3. light source –► polarizer –► solution of chiral molecules - Chiral media can rotate the plane of polarized light if the indices or refraction (velocities) of the two helical vectors of light are different. - Instructor talks about this in detail. Instructor stresses the fact that rotation of the plane of polarized light is not an exception to the rule that enantiomers have identical properties. - The extent of the rotation is dependent on path length in the chiral media and concentration of the chiral molecule. It is also dependent on wavelength in a way that is difficult to predict. Chem 230 Lecture 12 Page- 2 - - There is a constant that expresses the material’s native ability to rotate the plane of polarized light. i. α = [α]l c ii. where α is the observed rotation, [α] is the specific rotation (a constant with units degree●mL/(g●dm), l is the path length in dm and c is the concentration in g/mL. [α] only applies at wavelength = 598 nm, the sodium D line. 4. Instructor compares 2,3-dichlorobutanoic acid to 2,3-dichlorobutane - he found that there were four different isomers for 2,3-dichlorobutanoic and only three for 2,3-dichlorobutane - the later compound contains an internal mirror plane in the (R,S) diastereomer that is the two molecules on the bottom of the figure below are identical! - Meso structures have stereogenic atoms, but the structure has an internal mirror plane. The (RS) absolute configuration below is a meso structure. Cl Cl Cl Cl (2R, 3R)-1,2-dichlorobutane (2S, 3S)-1,2-dichlorobutane Cl Cl identical meso structures Cl 5. (2R, 3S)-1,2-dichlorobutane Cl (2S, 3R)-1,2-dichlorobutane 6. However the figure below contains four stereoisomers - all four of them are different. - the R,S structure no longer has an internal mirror plane. Cl Cl CO2H CO2H Cl (2R,3R)-1,2-dichlorobutanoic acid Cl Cl (2S,3S)-1,2-dichlorobutanoic acid Cl CO2H Cl 7. (2R,3S)-1,2-dichlorobutanoic acid CO2H Cl (2S,3R)-1,2-dichlorobutanoic acid Chem 230 Lecture 12 Page- 3 8. Biological compounds are usually found in the body as one enantiomer. - so even though we are fairly symmetrical on the outside we are not so on the inside physiologically or microscopically. - our hearts are on our left side, my mirror image has his heart on his right side i. What does this have to do with my pitcher/ batter scenario above? Does this fact screw up the scenario? How? - I have (S)-aminoacids (structure of an S amino acid appears below) in my proteins, my mirror image has (R)-aminoacids in his proteins. - At the level of atoms and molecules the mechanics of our internal organs are asymmetric. Biochemistry of all life on earth is chiral! NH2 O 9. OH - Valine, an amino acid. Can you find the stereogenic atom? Can you assign the absolute configuration of this stereogenic atom? 10.What is going on in the following scenario? - a racemic trans-1,2-diaminocyclohexane is treated with 1 equivalent (l+)-tartaric acid (R,R). A salt forms that contains only R,R-diamine and R,R-tartaric acid. - one of the diastereomeric pairs of salts has less solubility than the other - this can occur because diatereomers have different physical and chemical properties OH HO2C CO2H OH NH2 NH2 HO CO2(-) H3N NH2 NH2 HO CO2(-) H3N