Assigned problems for Chapter 10 on the
advertisement
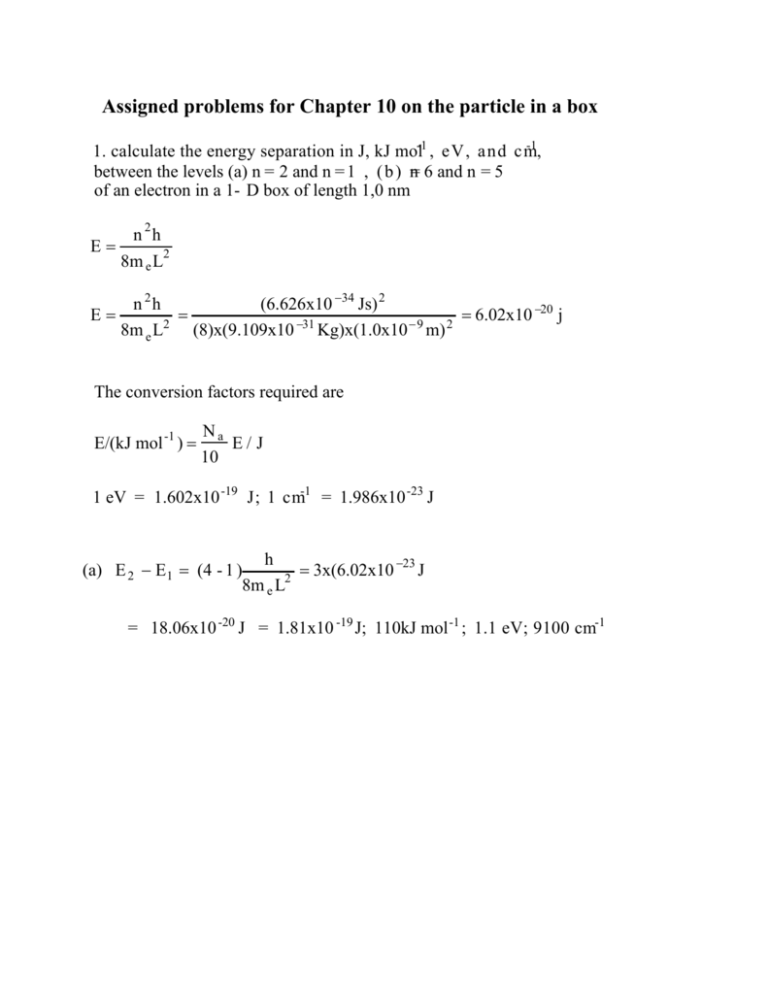
Assigned problems for Chapter 10 on the particle in a box -1 1. calculate the energy separation in J, kJ mol-1 , eV, and cm , between the levels (a) n = 2 and n = 1 , ( b ) n= 6 and n = 5 of an electron in a 1- D box of length 1,0 nm n 2h E= 8m e L2 E= n 2h (6.626x10 −34 Js) 2 = = 6.02x10 −20 j 2 −31 −9 2 8m e L (8)x(9.109x10 Kg)x(1.0x10 m) The conversion factors required are E/(kJ mol -1 ) = Na E/ J 10 1 eV = 1.602x10 -19 J; 1 cm-1 = 1.986x10 -23 J h −23 (a) E 2 − E1 = (4 - 1 ) J 2 = 3x(6.02x10 8m e L = 18.06x10 -20 J = 1.81x10 -19 J; 110kJ mol -1 ; 1.1 eV; 9100 cm-1 2. Calculate the probability that a particle will be found between 0.49 L and 0.51L in a 1 - D box of length L when it has (a) n = 1 , (b) n=2. The the wavefunction to be a constant in this range the wavefunctions are n = 2 n x sin L L The propability is 0.51L P= ∫ n 2 dx ≈ n 2 x 0.49L (a) P= 2 n x sin 2 x L L For x =.5L n= 1 P= 2 sin 2 x0.02L = 0.04 2 L 3. (a) Write the Schrödinger equation for a particle in a 1-D square well of length L (b) Calculate the expectation value of p and p 2 for a particle in the state n=1 Answ: (a) h2 d 2 − =E 2m dx 2 which has the solution n n x 2 sin L L = ˆp = − h d i dx L < pˆ >= ∫ 0 n n x 2h L n x d * pˆ n dx = 2 ∫ sin sin dx iL 0 L dx L 2h L n x n x = 2 ∫ sin cos dx = 0 L iL 0 L 4. What are the most likely locations of a particle in a box of length L in the state n = 3 ? 3 = P ( x )∝ 2 3 x sin L L 3 3 3 x ∝ sin 2 L The maxima and minima in P(x) corresponds to 3 x 3 x 6 x dP(x) ∝ sin cos ∝ sin L L L dx sin = 0 when 6 x = = n' , n = 0,1,2..... L which corresponds to x = n' L , n'< 6. 6 n' = 0,2,4, and 6 corresponds to minima in n' = 1, 3, and 5 to maxima dP(x) =0 dx 5. Set up the Schrödinger equation for a particle of mass m in a threedimensional square well with sides L x ; Ly ; Lz . Show that the wavefunction is defined by three quantum numbers and that the Schrödinger equantion is separable . Find the energy levels, and specialize the results to a cubic box of side L Please see handout on particle in 3-D box (Lecture-10) 6. To a crude first approximation, a electron in a linear polyene my be considered to be a particle in a one dimensional box. The polyene - carotene contains 22 conjugated C atoms, and the average internuclear distance is 140 pm. Each state up to n =11 is occupied by two electrons. Calculate (a) the separation in energy between the ground state and the first excited state in which one electron occupies the state with n=12. (b) The frequency of the radiation required to produce a transition between these two states (c) The total probability of finding an electron between C atoms 11 and 12 in the groundsate of the 22-electron molecule Answ: ( a ) : L =(21)x(1.40x10-10 m) = 2.94x10 −9 m E = E12 − E11 = h2 h2 = (2n + 1)x = (2x11 + 1)x 8mL2 8mL2 (23)x(6.626x10 −34 Js) = 1.603x10 (8)x(9.11x10 −31 Kg)x(2.94x10 −9 ) 2 −19 J = 1.60x10 −19 J (b) : E 1.603x10 −19 J = = = 2.42x10 14 s −1 = 2.42x10 14 Hz −34 h 6.626x10 Js 7. Consider a particle in a cubic box. What is the degeneracy of the levels that has an energy three times that of the lowest level.